Question
Question: A neutral compound A (molecular weight=74) on hydrolysis gives compounds (B) and (C). (B) reduces \(...
A neutral compound A (molecular weight=74) on hydrolysis gives compounds (B) and (C). (B) reduces HgCl2 whereas (C) gives an iodoform test. What are (A) (B) and (C)?
Solution
Hint : Iodoform test is a very useful test used to check the presence of carbonyl compounds with the structure R−CO−CH3 or alcohols with the structure R−CH(OH)−CH3 in a given unknown substance. Formic acid is a very good reducing agent that reduces mercurous chloride. Thus we need a compound that can produce formic acid and alcohol on hydrolysis.
Complete Step By Step Answer:
We are looking for a compound that can yield formic acid and an alcohol that can produce a positive result for the iodoform test. Thus we can say that the compound that we are looking for is ethyl formate that has a molecular formula of HCOOC2H5 . The structure of this compound is given as shown below:
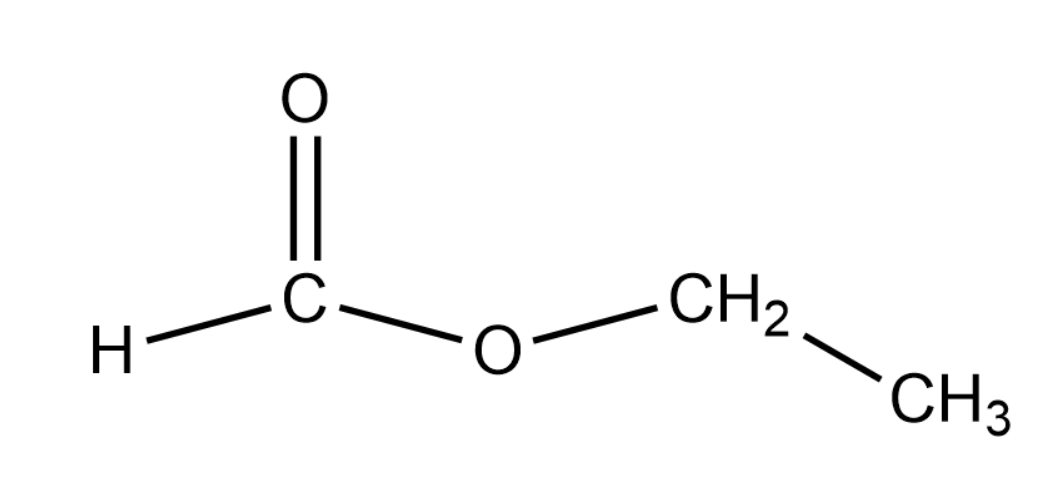
The reaction of the above compound with water is given the first step in the question. This is given by the following equation:
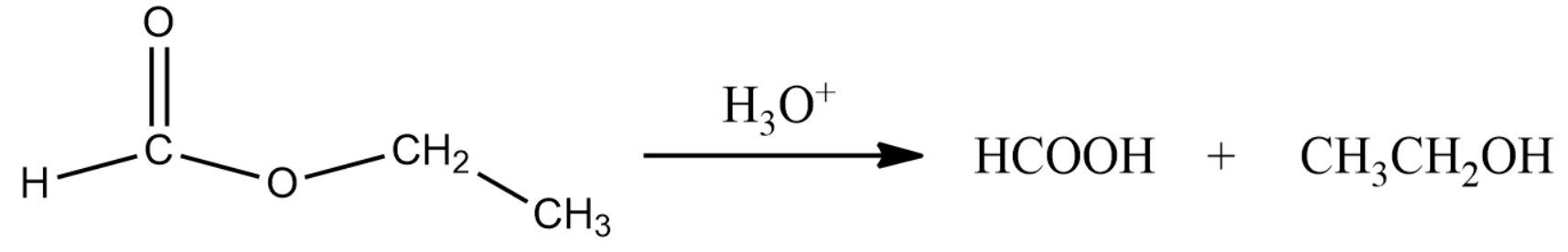
Formic acid when treated with HgCl2 will reduce the compound. Formic acid will turn into carbonic acid which in turn decomposes to carbon dioxide and water.
⇒HCOOH+2HgCl2→Hg2Cl2+CO2+2HCl
Ethanol on the other hand will give us a positive iodoform test. Ethanol forms acetaldehyde on oxidation, so it gives the iodoform test. The test is said to be positive when it forms a pale yellow precipitate when iodine in sodium hydroxide reacts with alcohol that can be oxidized to acetaldehyde is the iodoform reaction.
⇒CH3CH2OH + 3I2+ 4NaOH→CH3I(yellow ppt) + HCOONa + 5NaI + 5H2O
Thus compound (A) is ethyl formate, (B) is Formic acid and (C) is ethanol.
Note :
In questions like these make sure to work your way up. It will be easier to identify compounds that are giving iodoform tests and compounds that reduce mercurous chloride. This can then be used to find a compound that produces them by hydrolysis. If they provide you with the formula of the beginning compound then use that as a hint to find out the compound.
