Question
Question: A model for quantized motion of an electron in a uniform magnetic field $B$ states that the flux pas...
A model for quantized motion of an electron in a uniform magnetic field B states that the flux passing through the orbit of the electron is n(h/e) where n is an integer, h is Planck's constant and e is the magnitude of electron's charge. According to the model, the magnetic moment of an electron in its lowest energy state will be (m is the mass of the electron)
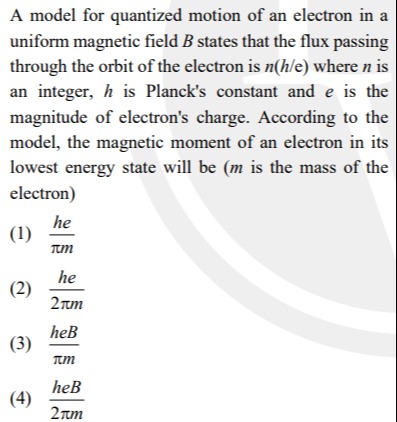
πmhe
2πmhe
πmheB
2πmheB
2πmhe
Solution
An electron moves in a uniform magnetic field B. The magnetic force provides the centripetal force for the circular motion: evB=rmv2, where e is the magnitude of the electron's charge, m is its mass, v is its speed, and r is the radius of the orbit. From this, we get v=meBr.
The magnetic flux Φ passing through the circular orbit of radius r is Φ=B⋅(Area)=B(πr2), assuming the magnetic field is perpendicular to the plane of the orbit.
According to the given model, the magnetic flux is quantized: Φ=n(h/e), where n is an integer, h is Planck's constant, and e is the magnitude of the electron's charge. So, B(πr2)=n(h/e). This gives r2=eBπnh.
The magnetic moment μ of a charged particle moving in a loop is given by μ=IA, where I is the current and A is the area of the loop. The area is A=πr2. The current I is the charge ∣−e∣=e passing a point per unit time. The time period of revolution is T=v2πr. The frequency of revolution is f=T1=2πrv. The current is I=ef=e2πrv.
The magnetic moment is μ=IA=(e2πrv)(πr2)=21evr.
Substitute the relation v=meBr into the magnetic moment formula: μ=21e(meBr)r=21me2Br2.
Now substitute the expression for r2 from the quantization condition: μn=21me2B(eBπnh). μn=2meBπe2Bnh=2mπenh.
This is the magnetic moment for the n-th state. The lowest energy state corresponds to the lowest possible integer value of n. Since the orbit exists (implying r=0), n must be a positive integer. The lowest positive integer is n=1.
For n=1, the magnetic moment in the lowest energy state is: μ1=2mπe(1)h=2πmeh.
Comparing this result with the given options, our result matches option (2).
