Question
Question: A mixture of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\), \({{\text{C}}_{\text{2}}}{{\text{H}...
A mixture of C2H6, C2H4 and C2H2 is bubbled through alkaline solution of copper (I) chloride, contained in Woulf’s bottle. The gas coming out is:
A.Original mixture
B.C2H6
C.C2H6 and C2H4 mixture
D.C2H4 and C2H2
Solution
Alkynes react with copper (I) chloride and form a red-brown coloured precipitate. Alkenes and alkanes do not react with copper (I) chloride.
Complete step by step answer:
When a mixture of ethyne (C2H2), ethene (C2H4) and ethane (C2H6) is bubbled through an alkaline solution of copper (I) chloride:
Ethyne (C2H2) reacts with the alkaline solution of copper (I) chloride.
Ethene (C2H4) and ethane (C2H6) do not react with the alkaline solution of copper chloride and come out as gas.
Thus, the gas coming out is C2H6 and C2H4 mixture.
Thus, the correct option is option (C).
Additional Information:
Ethane and ethene do not react with the alkaline solution of copper (I) chloride as they do not have acidic hydrogen.
Note: The reaction of ethyne (C2H2) with an alkaline solution of copper (I) chloride is as follows:
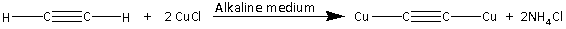
Copper (I) acetylide is produced in the reaction. Copper (I) acetylide is a brown colored precipitate.
