Question
Question: A mixture of $NH_3(g)$ and $N_2H_4(g)$ is placed in a sealed container at 300 K. The total pressure ...
A mixture of NH3(g) and N2H4(g) is placed in a sealed container at 300 K. The total pressure is 0.5 atm. The container is heated to 1200 K at which time both substances decompose completely according to the equations
2NH3(g)⟶N2(g)+3H2(g)
and
N2H4(g)⟶N2(g)+2H2(g)
After decomposition is complete, the total pressure at 1200 K is found to be 4.5 atm. Find the mole% of N2H4 in the original mixture.
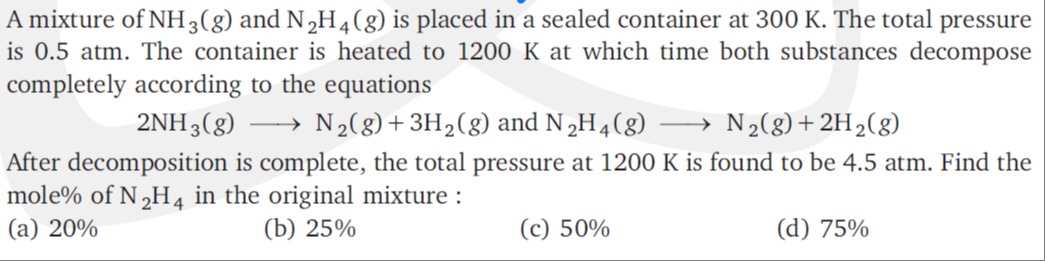
A
20%
B
25%
C
50%
D
75%
Answer
25%
Explanation
Solution
Let initial moles of NH₃ and N₂H₄ be x and y.
- Initial total moles: n = x + y.
- Initial pressure at 300 K: P₁ ∝ n·300.
- On decomposition at 1200 K:
• NH₃ → produces 2 moles gas per mole NH₃ ⇒ 2x
• N₂H₄ → produces 3 moles gas per mole N₂H₄ ⇒ 3y
Total final moles = 2x + 3y. - Final pressure at 1200 K: P₂ ∝ (2x+3y)·1200.
Since volume and R cancel,
More directly:
P1=0.5 atm,P2=4.5 atm⇒P1P2=9 (x+y)300(2x+3y)1200=9⟹x+y2x+3y=49=2.25 2x+3y=2.25(x+y)⟹2x+3y=2.25x+2.25y⟹0.75y=0.25x⟹y=3xMole fraction of N₂H₄ = y/(x+y) = (x/3)/(4x/3) = 1/4 = 25%.
