Question
Question: A mixture of NH3(g) and N2H4 (g) is placed in a sealed container at 300 K. The pressure within the c...
A mixture of NH3(g) and N2H4 (g) is placed in a sealed container at 300 K. The pressure within the container is 0.6 atm. When the container is heated to 1000 K, where the two gases undergo decomposition reactions as given below
2NH3(g) → N2(g) + 3H2(g) and N₂H₄(g) → N2(g) + 2H2(g)
The pressure of the container after complete decomposition becomes 4.8 atm. The mole percent of NH3(g) in the original mixture was x. The value of 10x is
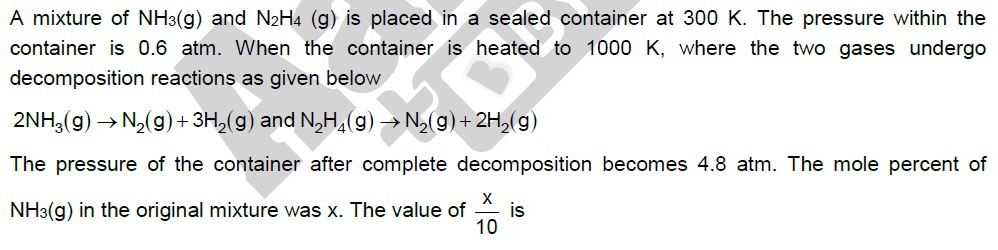
6
Solution
Let nNH3 and nN2H4 be the initial number of moles of NH3(g) and N2H4(g) in the sealed container. The initial total number of moles is ninitial=nNH3+nN2H4. The initial pressure is Pinitial=0.6 atm at Tinitial=300 K. The final temperature is Tfinal=1000 K, and the final pressure is Pfinal=4.8 atm. The volume of the container V is constant.
Using the ideal gas law, PV=nRT. For the initial state: PinitialV=ninitialRTinitial 0.6V=(nNH3+nN2H4)R(300) (1)
When the container is heated to 1000 K, the gases decompose completely according to the reactions:
-
2NH3(g)→N2(g)+3H2(g) From this reaction, 2 moles of NH3 produce 1+3=4 moles of gas. Thus, 1 mole of NH3 produces 2 moles of gas. If initially there were nNH3 moles of NH3, they will produce 2nNH3 moles of gas after decomposition.
-
N2H4(g)→N2(g)+2H2(g) From this reaction, 1 mole of N2H4 produces 1+2=3 moles of gas. If initially there were nN2H4 moles of N2H4, they will produce 3nN2H4 moles of gas after decomposition.
The total number of moles after complete decomposition is nfinal=2nNH3+3nN2H4. For the final state: PfinalV=nfinalRTfinal 4.8V=(2nNH3+3nN2H4)R(1000) (2)
Divide equation (2) by equation (1): 0.6V4.8V=(nNH3+nN2H4)R(300)(2nNH3+3nN2H4)R(1000) 8=(nNH3+nN2H4)×300(2nNH3+3nN2H4)×1000 8=3(nNH3+nN2H4)10(2nNH3+3nN2H4) 24(nNH3+nN2H4)=10(2nNH3+3nN2H4) 24nNH3+24nN2H4=20nNH3+30nN2H4 4nNH3=6nN2H4 2nNH3=3nN2H4
We are given that the mole percent of NH3(g) in the original mixture was x. Mole percent of NH3 = nNH3+nN2H4nNH3×100=x.
From the relationship 2nNH3=3nN2H4, we can express nN2H4 in terms of nNH3: nN2H4=32nNH3.
Substitute this into the mole percent expression: x=nNH3+32nNH3nNH3×100 x=(1+32)nNH3nNH3×100 x=(33+2)nNH3nNH3×100 x=(35)nNH3nNH3×100 x=53×100 x=60
The question asks for the value of 10x. 10x=1060=6.
