Question
Question: A mixture of ethyl iodide and n-propyl iodide is subjected to wurtz reaction. The hydrocarbon which ...
A mixture of ethyl iodide and n-propyl iodide is subjected to wurtz reaction. The hydrocarbon which will be majorly formed is:
A. Butane
B. Propane
C. Pentane
D. Hexane
Solution
Two different or same alkyl halides undergo dimerization in presence of sodium metal is known as Wurtz reaction. This reaction is also called a coupling reaction because the two alkyl halides combine (couples) and form a normal hydrocarbon chain as the product.
Complete step by step answer:
- In the given question the reactants are ethyl iodide and n-propyl iodide.
- The above reactants are subjected to wurtz reaction means reacted in presence of sodium metal.
- The structures of the ethyl iodide and n-propyl iodide are as follows.
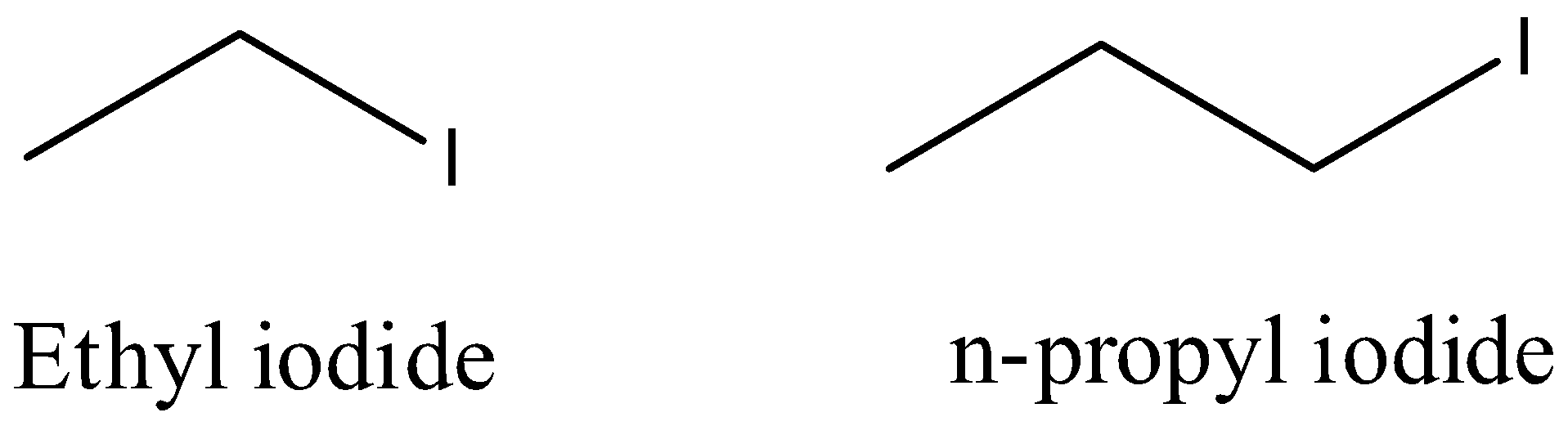
- The reaction of ethyl iodide with n-propyl iodide in presence of sodium metal is as follows.
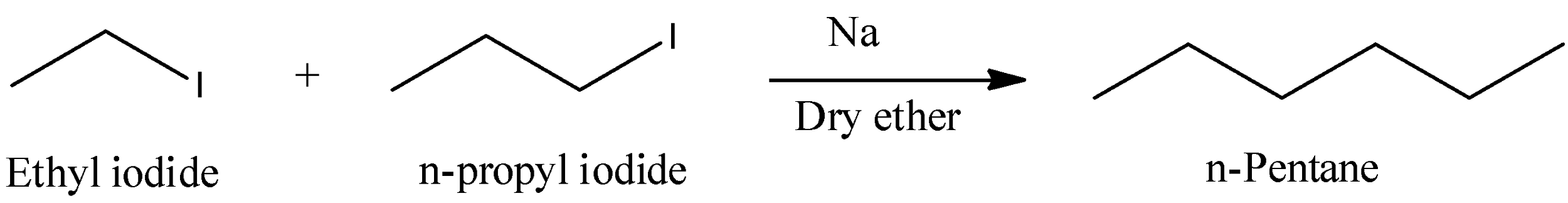
- In the above reaction ethyl iodide couples with n-propyl iodide in presence of sodium metal and forms n-pentane as the product.
- Therefore the product formed when a mixture of ethyl iodide and n-propyl iodide is subjected to wurtz reaction is n-pentane.
So, the correct answer is “Option C”.
Note: In wurtz reaction dry ether acts as a solvent to make the reactants with one another and makes the sodium metal to not react with moisture present in the air. Wurtz reaction has to be carried out in dry medium because sodium metal is highly reactive towards the moisture. Therefore we have to use dehydrated solvents to carry out the wurtz reaction. Generally Wurtz reaction is used to prepare higher alkanes in organic chemistry.
