Question
Question: A mixture contains two salts which gives following results. ...
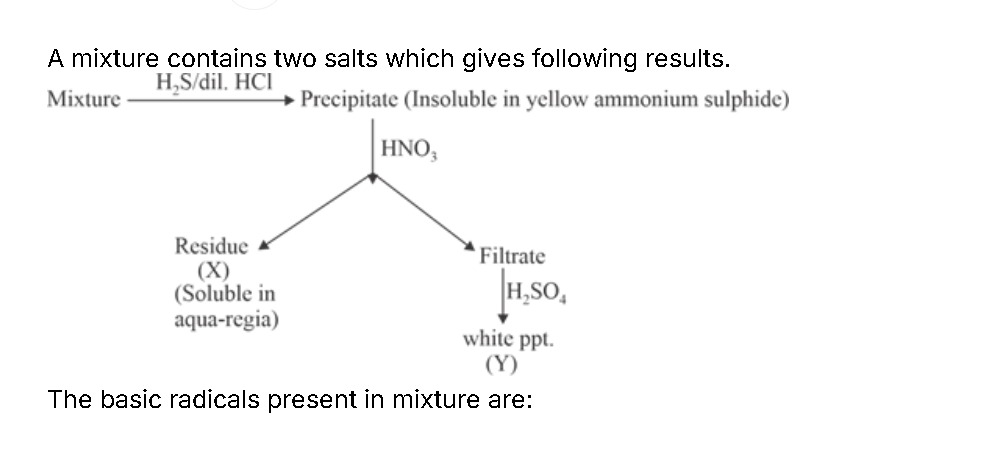
A mixture contains two salts which gives following results.
Bi³⁺ and Pb²⁺
Solution
-
Precipitation step:
On treating the mixture with H₂S in dilute HCl, two types of sulfides can form. However, note that PbS dissolves in (yellow) ammonium sulfide, while Bi₂S₃ does not.- Thus, Bi³⁺ → Bi₂S₃ (insoluble in ammonium sulfide).
-
Acid treatment:
When the precipitate is treated with HNO₃, the Bi₂S₃ (insoluble in ammonium sulfide) remains as an insoluble residue (X). In contrast, the other salt (from Pb²⁺) had not precipitated as its sulfide because PbS dissolves in ammonium sulfide; hence Pb²⁺ remains in solution (filtrate). -
Sulfate precipitation:
Addition of H₂SO₄ to the filtrate brings about the precipitation of PbSO₄ as a white solid (Y). This confirms the presence of Pb²⁺ in the filtrate. -
Confirmation of residue:
The residue (X), which is Bi₂S₃, dissolves in aqua regia – a property characteristic of bismuth compounds.
Thus, the two basic radicals present (i.e. the cations) in the original mixture are Bi³⁺ and Pb²⁺.
