Question
Question: A metal M and its compounds can give the observable changes as shown in the above sequence of reacti...
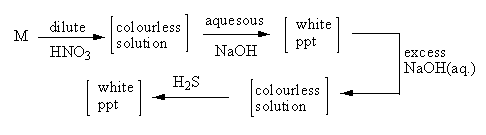
A metal M and its compounds can give the observable changes as shown in the above sequence of reactions. The metal M is
A. Mg
B. Pb
C. Zn
D. Sn
Solution
As here, four metals are given and we have to identify the products and the reaction of metal with acid gives hydrogen gas. So, we should have some idea about the electrochemical series, so we can decide which metal will react with acid. We should also have ideas about solubility rules, so we can decide whether a solution is forming or precipitating.
Complete solution:
Let’s start with option A, Mg so, Mg will react with nitric acid and form magnesium (II) nitrate. The reaction is shown as follows:
Mg + HNO3→magnesiumnitrateMg(NO3)2 + H2
Here, the Mg is in (II) oxidation state so, the electronic configuration of Mg2 + is 1s22s22p6. So, magnesium ion has a fully-filled electronic configuration and d-orbital does not split due to ligand field, so the solution of magnesium (II) nitrate will be colourless.
When magnesium (II) nitrate reacts with aqueous sodium hydroxide, the nitrate ions get replaced by hydroxide ions. The reaction is shown as follows:
Mg(NO3)2 + aq.NaOH→magnesiumhydroxideMg(OH)2+2NaNO3
Hydroxides are insoluble in water, so the magnesium hydroxide will precipitate and the colour of this precipitate is white.
When magnesium hydroxide reacts with an excess of aq. Sodium hydroxide, the reaction goes in a backward direction, so no colourless solution forms because magnesium hydroxide on dissociation gives hydroxide ion and aq. NaOH also gives hydroxides ions so, due to the common ion effect, the reaction goes in a backward direction and no product forms. So, here, we can eliminate option A.
Now let’s start with Pb metal, Pb also forms lead (II) nitrate with nitric acid. Nitrate of lead is insoluble so, a colourless will not form. The reaction of Pb with nitric acid gives a white precipitate of lead (II) nitrate so, here, we can eliminate the option B.
Similar to Mg and Pb, Zn also form zinc nitrate with nitric acid. Zinc is present as Zn2 + ion so, zinc ion has fully-filled d-orbitals so, it does not show colour and nitrates are soluble so, zinc nitrate is a colourless solution.
Zn + HNO3→zincnitrateZn(NO3)2 + H2
Zinc nitrate reacts with aq. NaOH to give zinc hydroxide the reaction is as follows:
Zn(NO3)2 + aq.NaOH→zinchydroxideZn(OH)2+2NaNO3
Zinc hydroxide is insoluble in water, so zinc hydroxide precipitates as a white solid powder.
When zinc hydroxide reacts with an excess of aq. NaOH to give sodium zincate. The reaction is as follows:
Zn(OH)2 + excessaq.NaOH→sodium zincateNa2ZnO2+H2O
Sodium zincate is water soluble and zinc is also in (II) oxidation state, so it is a colourless solution.
When sodium zincate reacts with hydrogen sulphide, it gives the zinc sulphide and sodium hydroxide. The reaction is as follows:
sodium zincateNa2ZnO2 + H2S→zincsulphideZnS + NaOH+H2O
Zinc sulphide is insoluble in water, so it precipitates and the colour of the precipitate is white so, the reaction of sodium zincate with hydrogen sulphide gives a white precipitate. So, option C is correct.
The reaction of Sn with nitric acid gives stannic oxide. The reaction is as follows:
Sn + 4HNO3→stannic oxideH2SnO3 + 4NO2 + H2O
Stannic oxide is colourless solid. So, the reaction of Sn with nitric acid does not give a colourless solution, so we can eliminate option D.
Therefore, the correct answer is (C).
Note: According to the solubility rules, nitrates are soluble except nitrate of lead, barium, and mercury. Oxides and hydroxides are insoluble. The insoluble compound precipitates. The metal ion having a fully-filled orbital does not cause colour, so zinc nitrate is colourless. Zinc sulphide is coloured due to ligand to metal charge transfer.
