Question
Question: A metal crystallizes in bcc lattice. The percent fraction of edge length not covered by atom is- A...
A metal crystallizes in bcc lattice. The percent fraction of edge length not covered by atom is-
A 10.4 %
B 13.4 %
C 12.4 %
D 11.4 %
Solution
A Body-centered cubic lattice (bcc) unit cell has atoms at each corner of the cube and an atom at the centre of the structure. The atom at the body centre wholly belongs to the unit cell in which it is present.
Complete Step by step answer: In Body-centered cubic lattice (bcc) unit cell every corner has atoms has a open structure and we have - 8 corners × 1/8 per corner atom = 8 × 1/8 = 1 atom
1 body centre atom = 1 × 1 = 1 atom
Therefore, the total number of atoms present per unit cell =2 atoms .
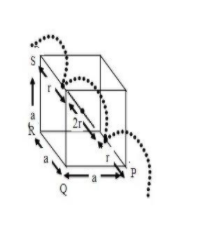
In body centered cubic lattice, the atom at the body centre touches the spheres at the corners .The body diagonal has a length that is four times the radius of the atomr
Body diagonal PS =4r,
Face diagonal PR=PQ2+QR2=2a
Body diagonal =PS=PR2+RS2=2a2+a2=3a
radius of atom(r) ,
(divide both the sides by 2)
Edge length of bcc is \to $$$$\;a
Length of two atoms → 2r
∴ Edge length not covered by atom =a−2r = a −23a=a2(2−3)
∴% fraction not covered = aa2(2−3)×100=22−1.732×100 = 13.4%
so the option (B) is correct.
Note: In Body-centered cubic lattice (bcc) unit cell vectors a = b = c and interaxial angles α=β=γ=90°, There are 23 metals that have the bcc lattice like it include iron, chromium, tungsten, tantalum, and molybdenum. etc.
