Question
Question: A metal chloride contains 55.0% of chlorine by weight 100 mL vapours of the metal chloride at STP we...
A metal chloride contains 55.0% of chlorine by weight 100 mL vapours of the metal chloride at STP weight 0.57g. The molecular formula of the metal chloride is
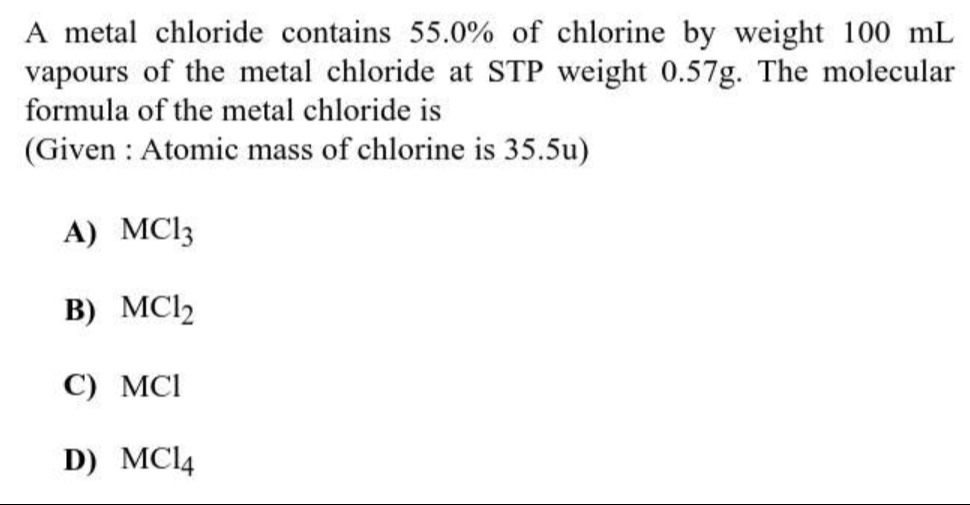
A
MC13
B
MC12
C
MCI
D
MC14
Answer
MC12
Explanation
Solution
-
Calculate the molar mass of the metal chloride: Molar Mass = (Weight of vapor / Volume of vapor) * Molar Volume at STP Molar Mass = (0.57 g / 100 mL) * 22400 mL/mol = 127.68 g/mol
-
Calculate the mass of chlorine in one mole: Mass of Cl per mole = Molar Mass * (% of Cl / 100) Mass of Cl per mole = 127.68 g/mol * (55.0 / 100) = 70.224 g/mol
-
Determine the number of chlorine atoms: Number of Cl atoms = Mass of Cl per mole / Atomic mass of Cl Number of Cl atoms = 70.224 g/mol / 35.5 g/mol ≈ 1.978 ≈ 2
-
The molecular formula is MCl₂.
