Question
Question: A metal ball of mass 1 kg is kept in a room at temperature 30°C. The heater supplies heat to the bal...
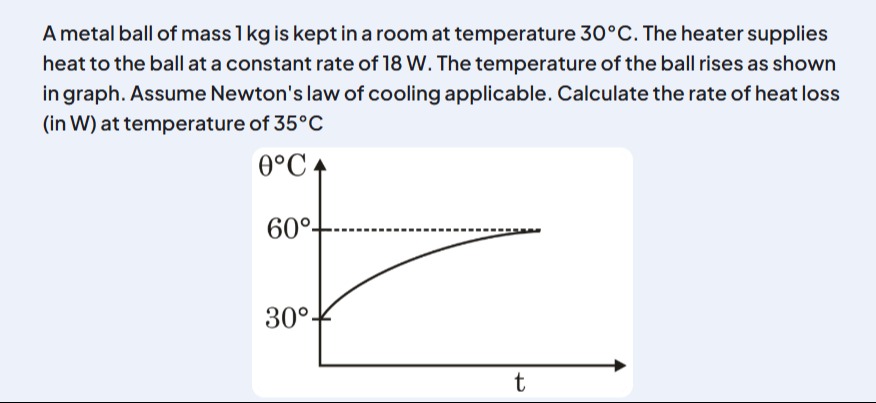
A metal ball of mass 1 kg is kept in a room at temperature 30°C. The heater supplies heat to the ball at a constant rate of 18 W. The temperature of the ball rises as shown in graph. Assume Newton's law of cooling applicable. Calculate the rate of heat loss (in W) at temperature of 35°C
Answer
3 W
Explanation
Solution
-
At equilibrium (60°C), the heat supplied equals the heat lost. Thus, by Newton’s law of cooling:
Heat loss rate=k(60−30)=18 WSo,
k=3018=0.6W/°C -
At 35°C, the temperature difference is:
35−30=5°CThen, the rate of heat loss is:
Heat loss rate=0.6×5=3WExplanation (Minimal):
- Equilibrium gives k=0.6W/°C.
- At 35°C, heat loss =0.6×(35−30)=3W.
