Question
Question: A mercury lamp is a convenient source for studying frequency dependence of photoelectric emission, s...
A mercury lamp is a convenient source for studying frequency dependence of photoelectric emission, since it gives a number of spectral lines ranging from the UV to the red end of the visible spectrum. In our experiment with rubidium photo-cell, the following lines from a mercury source were used
λ1=3650A,λ2=4358A,λ3=4358A,λ4=5461A,λ5=6907A.
The stopping voltages, respectively, were measured to be:
V01=1.28V,V02=0.95V,V03=0.74V,V04=0.16V,V05=0V,
Determine the value of Planck's constant h, the threshold frequency and work function for the material.
Solution
Plot a graph of Voltage vs Frequency with the values given above. The slope of the curve is eh and makes an intercept ehν0 on the negative. Substitute the charge of the electron to obtain Planck's constant and further find the threshold frequency and work function.
Complete step by step answer:
We have the values of Voltage but we need to find the values of frequency for the corresponding readings.
Formula to find frequency:ν=λc
Where,
c is speed of light,
λ is the wavelength of light.
Substituting the values of c and λ to obtain the values of ν.
ν1=λ1c=3650∗10−103∗108=8.22∗1014Hzν2=λ2c=4047∗10−103∗108=7.41∗1014Hzν3=λ3c=4358∗10−103∗108=6.88∗1014Hzν4=λ4c=5461∗10−103∗108=5.49∗1014Hzν5=λ5c=6907∗10−103∗108=4.34∗1014Hz
Plotting a graph of V0vs ν:
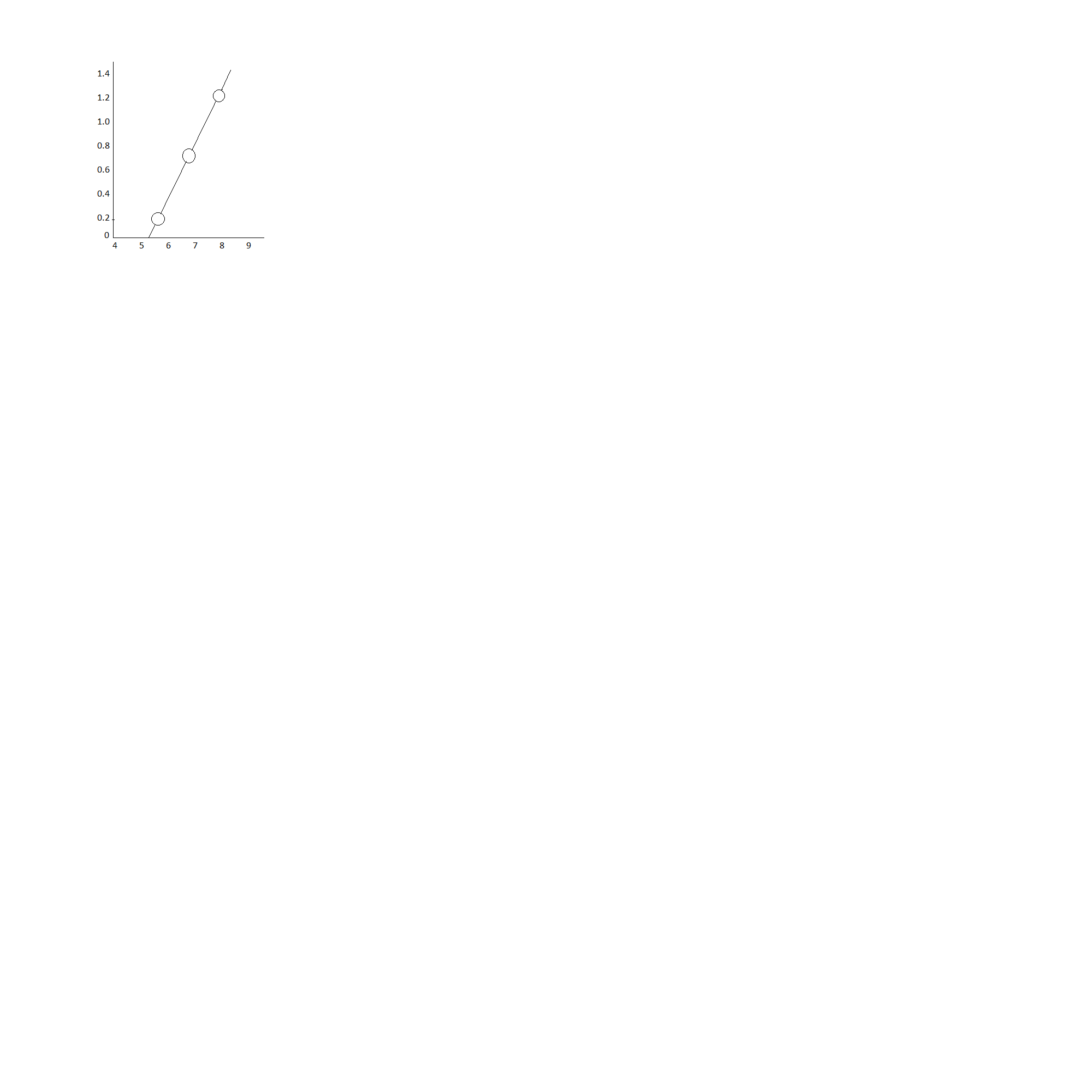
In the graph given, the X axis represents the values of
ν(x1014), and the Y axis represents the value of V0.
From Einstein's equations of photoelectric effect, we have
hν=hν0+21mv2max
If V0 is the stopping potential, then we have
eV0=21mv2max
hν=hν0+eV0
Or V0=ehν−ehν0
The above equation represents a straight line whose slope is eh and makes and intercept ehν0 with negative y axis.
From the above formula we can calculate slope of the graph,
8.22∗1014−5.49∗10141.28−0.16=4.1∗10−15JsC−1
