Question
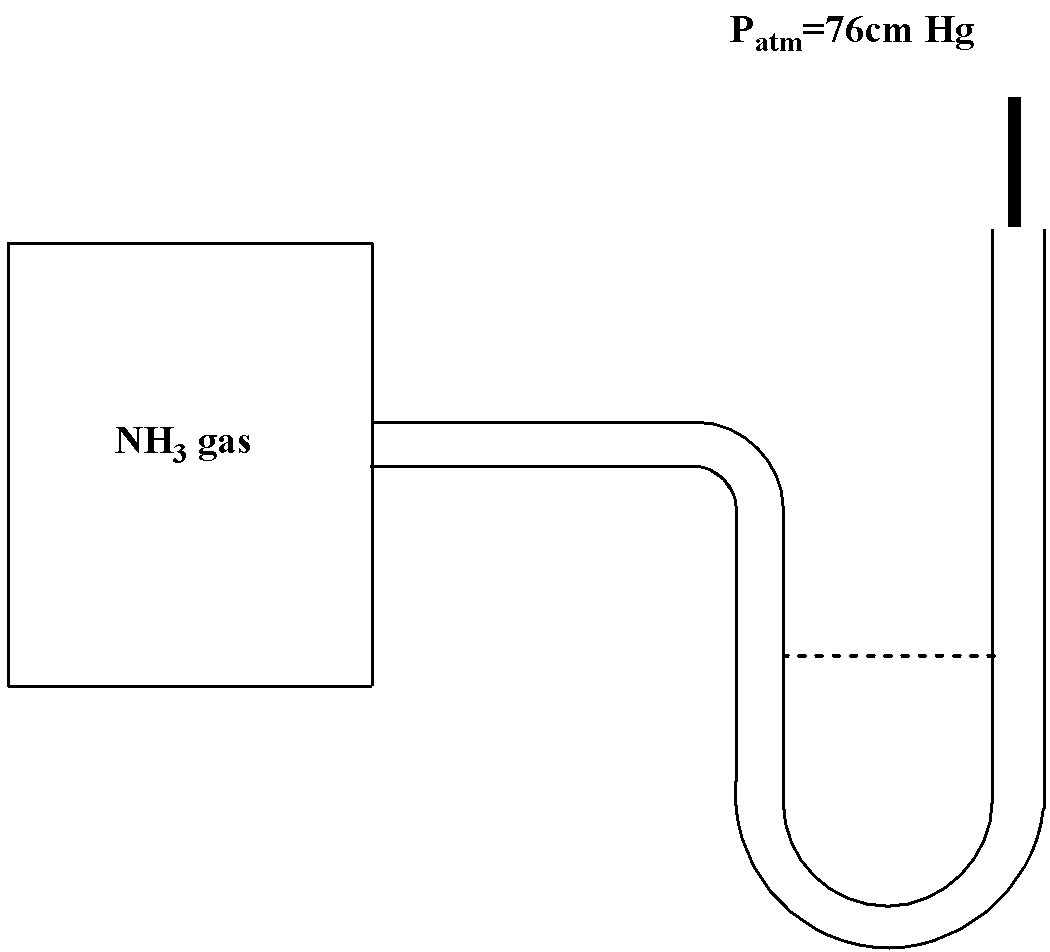
Question: A manometer attached to a flask containing ammonia gas has no difference in mercury level initially ...
A manometer attached to a flask containing ammonia gas has no difference in mercury level initially as shown in diagram. After sparking into the flask, ammonia is partially dissociated as 2NH3(g)→N2(g)+3H2(g) now it have difference of 6 cm in mercury level in two columns, what is partial pressure of H2(g) at equilibrium?
(A) 6 mm Hg
(B) 18 mm Hg
(C) 27 mm Hg
(D) None of these
Solution
According to ideal gas law, partial pressure is inversely proportional to volume. It is directly proportional to the number of moles and temperature. Then the pressure at initial and pressure at equilibrium will be found.
Complete step by step answer:
We have been given the question that a manometer attached to the flask contains ammonia gas.
Then we have been given that ammonia is partially dissociated to nitrogen gas and hydrogen gas as follows
2NH3(g)→N2(g)+3H2(g)
It is given that ammonia is at 76 cm Hg pressure. Therefore the initial pressure will be 76 cm Hg
At equilibrium, the pressure will be 76−2x,x,3x respectively. So the total pressure will be written as 76−2x+x+3x=76+2x
So the total pressure at equilibrium is 76+2x
We can observe from here that the increase in pressure is 76−(76+2x)=2x
It is given that difference in mercury level is six cm
⇒2x=6
⇒x=3 cm
So the partial pressure of hydrogen gas =3x
⇒3×3=9cmHg
So the partial pressure of hydrogen is 9cmHg
So, the correct answer is Option D.
Additional information:
The difference between partial pressure and vapor pressure is that partial pressure is the pressure exerted by the gas individually in the mixture. While vapor pressure is the pressure exerted by the liquid at the thermodynamic equilibrium.
Note: The partial pressure of an individual gas is equal to the total pressure multiplied by the mole fraction of that gas.
The partial pressure of gas = Total pressure × mole fraction of gas
⇒P=PTXA
Also, the total pressure of an ideal gas mixture is the sum of the partial pressure of the gases in the mixture. The pressure exerted by vapors of liquid is called vapor pressure.
