Question
Question: (a) List three properties of ionic compounds. (b) Show the formation of \[NaCl\] by transfer of el...
(a) List three properties of ionic compounds.
(b) Show the formation of NaCl by transfer of electron
(c) How is it that ionic compounds in the solid state do not conduct electricity but they do so when in molten state?
Solution
Hint : We must know the concepts of ionic compounds, their formation, characteristics are important to remember. Ionic compounds are compounds composed of ions, charged particles that form when an atom (or group of atoms) gains or loses electrons. (A cation is a positively charged ion; an anion is a negatively charged ion.)
Complete step by step solution :
(a) Properties of ionic compounds:
1. The ionic compounds are solids at room temperature. (For example NaCl and LiBr)
They are formed by gaining or losing electrons. If an element loses its electrons then it carries positive charge or if an element gain electrons then it carries negative charge
2. Ionic compounds usually soluble in water
They shows high melting and boiling point due to oppositely charged partials with strong electrostatic force of attraction
3. They are good conductors of electricity
They are easily soluble in water and give ions. So, the free ions help to conduct electricity.
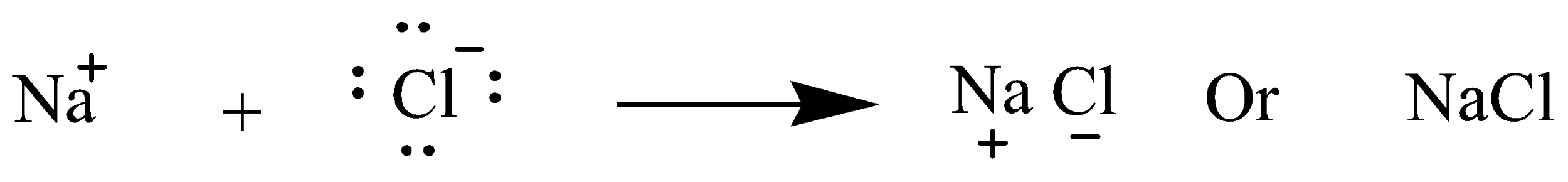
(b) Presentation of NaCl formation by transfer of electron
The atomic number of sodium(Na) is 11 so electrons present in the last shell are 1 whereas the atomic number of chlorine(Cl) is 17 so electrons present in the last shell is 7. So Sodium transfers or gives its 1 electron to chlorine and become octet stabilized.chem This is complete transfer of electrons so NaCl is ionic compound
(c ) In solid state, the ions in the compounds are not free to move and are held in crystal lattices, so the ions are unable to carry electricity. To conduct electricity, ions need to be free and moving. In molten state, they become mobile and thus are able to conduct electricity
Note : Different types of compounds are present, and the characteristics of compounds depend upon their type of bonds that they form. Ionic compounds are formed from ionic bonds, and so their properties are with respect to ionic bonds.
