Question
Question: A liquid will not wet the surface of a solid if its angle of contact is A. Zero B. Less than \({...
A liquid will not wet the surface of a solid if its angle of contact is
A. Zero
B. Less than 90∘
C. More than 90∘
D. 90∘
Solution
Relate the angel of contact with the wettability of the surface by using the concept of cohesive and adhesive forces. Also the wettability of the surface not only depends on contact angle, it also depends on molecular forces.
Complete step-by-step answer:
At the interface of a liquid and a solid, the angle between the surface of the liquid and the outline of the contact surface is defined as angle of contact (θ). This angle of contact (θ) is used for checking the wettability of solid surfaces.
This angel of contact and the wettability of the solid surface is also depend on two type forces which are:
1. Cohesive Force: the intermolecular attractive force between the same types of molecules are known as cohesive force.
2. Adhesive Force: the intramolecular attractive force between the different-different types of molecules is called an adhesive force.
Now, at the contact surface between liquid and solid these attractive forces are responsible for wetting.
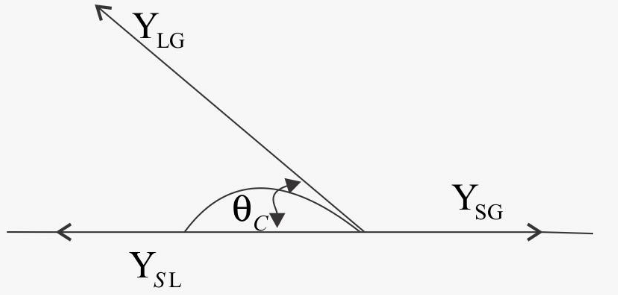
Here, many conditions for forces and angle of contact:
When, 0≤ angle of contact ≤ 90∘ adhesive force> cohesive force ……… (i)
And when angle of contact >90∘ cohesive force > adhesive force ……… (ii)
So, from equation (ii) the intermolecular attractive force (cohesive force) is larger than adhesive force hence there will be no holding force acting between the molecules of liquid and solid surface. Hence there will be no wetting in case of obtuse angle.
∴Option (C) is correct.
Note: This angle of contact completely depends on the properties of a particular liquid and contacting surface. Also it depends on the surface tension of the liquid. Surface tension is the property by which the force exerted by the intermolecular forces by unit length.
