Question
Question: A kettle with 2-litre water at \(27^{\circ} C\) is heated by an operating coil heater of power \(1kW...
A kettle with 2-litre water at 27∘C is heated by an operating coil heater of power 1kW. The heat is lost to the atmosphere at a constant rate of 160J/sec when its lid is open. In how much time will water be heated to 77∘C with the lid open? (specific heat of water= 4.0 kJ/kg)
Solution
According to the law of conservation of energy, the heat lost by a hot body is completely absorbed by a cold body. This flow of heat continues until the two bodies are in thermal equilibrium. This is known as the principle of calorimetry.
Complete step by step solution:
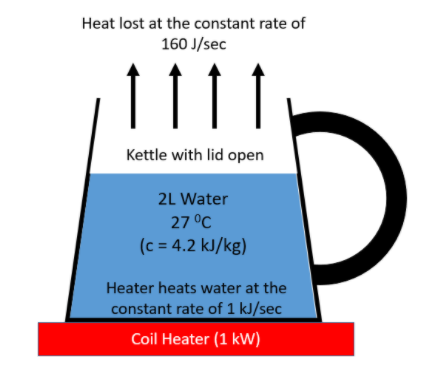
As seen in the diagram, there is a kettle with its lid open and containing 2 litres of water at 27∘C. The kettle is kept on a coil heater of power 1kW. This means the heater is capable of heating at a constant rate of 1kJ/sec or 1000J/sec. Since the lid is open, the water loses heat at a constant rate of 160J/sec as mentioned in the question.
Let us assume at a certain time T, the heater heats the water inside the kettle to a temperature of 77∘C. Therefore, the heat energy supplied by the coil heater until time T is
Eheater=(1000T)J ………. (1)
The water in the kettle absorbs the heat supplied by the heater. At time T, the water gets heated up and attains a temperature of 77∘C. Therefore, heat energy absorbed by the water until time T is given by
Ewater=m×c×Δθ ………. (2)
Where m is the mass of water present in the kettle, c is the specific heat capacity of water, and Δθ is the change in temperature of water (i.e. initial temperature subtracted from the final temperature).
Mass of water can be expressed in terms of volume and density as
m=V×ρ ………. (3)
Here, ρ is the density of water, which is 1 kg/L. Therefore
m=(2×1)kgm=2kg
Now, substituting the values of m, c, and Δθ in Eq. 2, we get
Ewater=2×4.2×103×(77−27)Ewater=42×104J
Heat lost due to open lid until time T is
Elost=160T ………. (4)
Now, by the law of conservation of energy, energy supplied by the heater until time T must be equal to the sum of energy absorbed by water and energy lost due to the absence of lid. Therefore,
Eheater=Ewater+Elost⇒1000T=42×104+160T⇒840T=42×104⇒T=500sec⇒T=8min20sec
∴ The heater will take 8 minutes 20 seconds to heat the water to 77∘C.
Note:
In this question, the specific heat capacity of the material of the kettle was neglected. In the former case, the kettle will also absorb heat supplied from the heater and has to be considered while performing the calculations.
