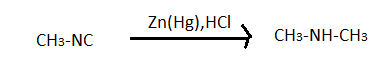Question
Question: (A) is subjected to reduction with \(Zn - Hg/HCl\) and the product formed is N-methylmethanamine. (A...
(A) is subjected to reduction with Zn−Hg/HCl and the product formed is N-methylmethanamine. (A) can be:
A. Ethane nitrile
B. Nitroethane
C. Carbylamine ethane
D. Carbylamino methane
Solution
Hint : We can solve this problem with the help of Clemmensen Reduction reaction.
Complete step by step solution:
As it is given in the problem statement that (A) is subjected to reduction with Zn−Hg/HCl so it is clearly a clemmensen reduction reaction. In this reaction aldehydes and ketones (carbonyl group) with zinc amalgam (Zn/Hg) in concentrated hydrochloric acid reduce the aldehydes or ketone to a hydrocarbon. The mercury alloy does not participate in the reaction it just used to provide a clean active metal surface.
This reduction involves heating a carbonyl compound with finely divided amalgamated zinc in hydroxylic solvent which contains a mineral acid such as hydrochloric acid. In clemmensen reduction nitro group is also reduced in presence of zinc amalgam and concentrated hydrochloric acid but nitro group containing compounds reduces very slowly. This reaction reduces RCN to amine (RCH2NH2) and isocyanide compounds to RNHCH3. Here in the problem it is given that the product is N-Ethylmethylamine. So it is clear that there is a isocyanide group in the A and R is methyl group CH3− as it gives a compound which has only two carbon.
A→CH3−NH−CH3 ⇒So know we know that the compound (A) is CH3−NC which is carboxyl amino methane.
Hence the last option, that is option D is the correct answer to this problem.
Note : : We have approached this reaction with clemmensen reduction. In this reaction it reduces carbonyl group to simple hydrocarbons. Thus the compound A is here CH3−NC which carbylamine methane. In the problem the product is given which makes it very easy for us to determine compound (A) as we know that compound that contains iso cyanide group give such type products.