Question
Question: A is named as: 
A) [R]−1−ethylcyclohex−2−enol
B) [S]−1−ethylcyclohex−2−enol
C) S−3−ethylcyclohex−3−en−3−ol
D) None of these
Solution
The compound can be named based on IUPAC rules. The general presentation of the organic compound is as follows:
Substituents + Word root + primary suffix + secondary suffix
If the compound contains the chiral carbon atoms, then the stereochemistry at the chiral carbon atom is expressed as R or S configuration. It depends on the properties assigned to the substituents.
Complete answer:
We are interested in determining the name of the organic compound. We will follow the two-step to determine the name of the compound
- IUPAC nomenclature
- Stereochemistry or the configuration at the chiral centre
Step 1) IUPAC nomenclature:
The compound is given as follows:
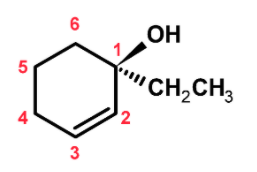
The compound has a six-membered ring. The word root would be hexane. Since it is a ring we add the ‘cyclo’ before the hexane word root.
The ring has a substituent such as a hydroxyl group −OH and an ethyl group −CH2CH3 . The −OH group is treated as the primary functional group and written as a secondary suffix. The secondary suffix is added to the work root. Thus we add ‘-ol’ as the secondary suffix. The compound contains the double bond at the 2 number position. It is treated as a primary suffix. The ethyl group is treated as a substituent. The IUPAC name of the compound is,
Substituents +Cyclo + Word root + primary suffix + secondary suffix
The name is,
1−Ethyl−cyclohex−2−enol
Step 2) now will find out the configuration at the chiral centre. The compound 1−Ethyl−cyclohex−2−enol has a chiral centre at the C1 carbon position. The C1 carbon atom is bonded to the −OH , −CH2CH3 and two carbon atom on either side of it.
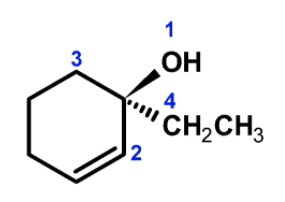
To determine the configuration, - First assign the properties to the groups on the chiral carbon atom. The priorities are assigned based on the atomic number of the directly bonded atom. The oxygen of the hydroxyl group has a higher atomic number than the other groups. Thus the −OH has given the (1) propriety .the other priorities are as shown in the following figure,
Now, draw an arrow starting from the highest priority group to the lowest priority group.
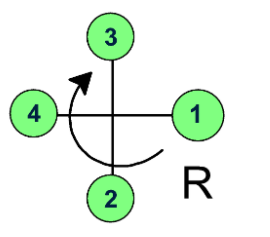
Here, the arrow goes through the clockwise direction, the chiral carbon has the R configuration.
Thus the name of the compound A is [R]-1-methylcyclohex-2-enol.
Hence, (A) is the correct option.
Note: The R and S, when the lowest priority group is towards the observer (wedge) then the configuration obtained is changes form R→ S orS→ R. If the lowest priority group is away from the observer (dashed), then the configuration remains as it is. Here, the lowest priority group is away from the observer thus no change in the configuration.
