Question
Question: a. Illustrate the following reactions giving a suitable example for each? i. Decarboxylation b. ...
a. Illustrate the following reactions giving a suitable example for each?
i. Decarboxylation
b. Give simple tests to distinguish between the following pairs of compounds?
i. Pentan-2-one and pentan-3-one
ii. Benzaldehyde and acetophenone
iii. Phenol and benzoic acid
Solution
The reaction in which carbon dioxide is lost from the carboxylic acid is known as decarboxylation. Benzaldehyde has an aldehyde functional group whereas acetophenone has a methyl ketonic group. We have to identify the test used to test the presence of methyl ketones.
Complete step by step solution:
a. i. Decarboxylation:
The reaction in which carbon dioxide is lost from the carboxylic acid is known as decarboxylation.
Example of decarboxylation is as follows:
When sodium salt of carboxylic acid is heated with soda-lime carbon dioxide is lost. In this reaction, hydrocarbon is formed.
The decarboxylation reaction is as follows:
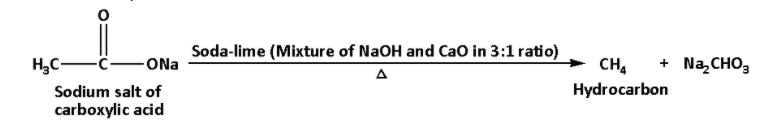
b. i. Chemical test to distinguish between pentan-2-one and pentan-3-one:
Pentan-2-one has a methyl ketone and pentan-3-one is not a methyl ketone.
The structures of pentan-2-one and pentan-3-one are as follows:
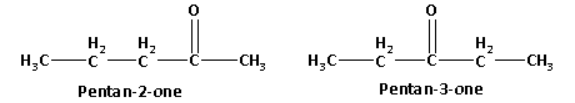
Pentan-2-one on reaction with sodium hypoiodite forms a yellow coloured precipitate of iodoform. The reaction of pentan-2-one with sodium hypoiodite is as follows:
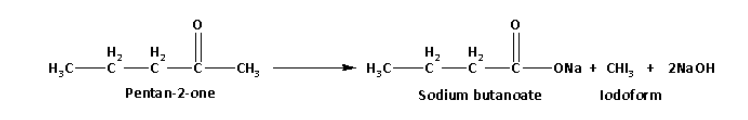
Pentan-3-one on reaction with sodium hypoiodite does not form a yellow coloured precipitate.
Thus, the reaction with sodium hypoiodite is the distinguishing test between pentan-2-one and pentan-3-one. This test is known as the iodoform test.
Thus, the chemical test to distinguish between pentan-2-one and pentan-3-one is the iodoform test.
ii. Chemical test to distinguish between benzaldehyde and acetophenone:
Benzaldehyde has an aldehydic functional group and acetophenone is a methyl ketone.
The structures of benzaldehyde and acetophenone are as follows:
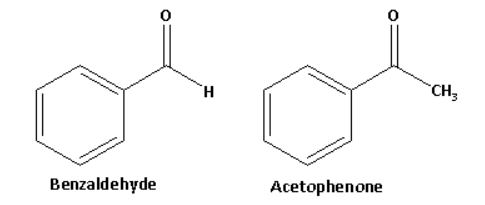
Acetophenone in reaction with sodium hypoiodite forms a yellow coloured precipitate of iodoform. The reaction of acetophenone with sodium hypoiodite is as follows:
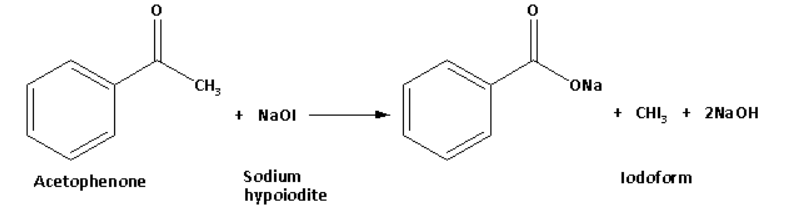
Benzaldehyde on reaction with sodium hypoiodite does not form a yellow coloured precipitate.
Thus, the reaction with sodium hypoiodite is the distinguishing test between benzaldehyde and acetophenone. This test is known as the iodoform test.
Thus, the chemical test to distinguish between benzaldehyde and acetophenone is the iodoform test.
iii. Chemical test to distinguish between phenol and benzoic acid:
The structures of phenol and benzoic acid are as follows:
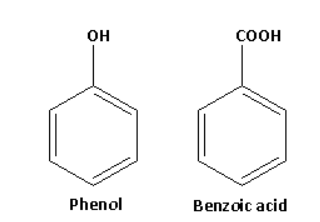
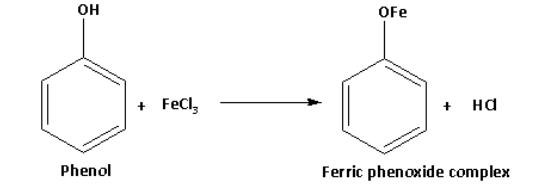
Phenol on reaction with neutral ferric chloride forms ferric phenoxide complex and gives violet colour. The reaction of phenol with neutral ferric chloride is as follows:
Benzoic acid on reaction with neutral ferric chloride forms ferric benzoate and gives buff coloured precipitate. The reaction of benzoic acid with neutral ferric chloride is as follows:
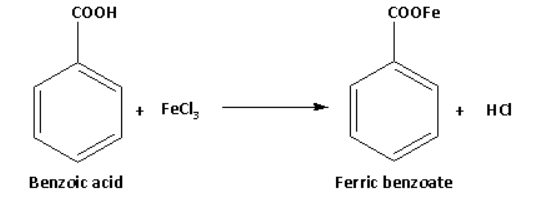
Thus, the reaction with neutral ferric chloride is the distinguishing test between phenol and benzoic acid. This test is known as neutral FeCl3 test.
Thus, the chemical test to distinguish between phenol and benzoic acid is the neutral FeCl3 test.
Note:
1. Methyl ketones give a positive iodoform test.
2. Neutral FeCl3 test is used to distinguish between phenol and benzoic acid.
