Question
Question: Na dry ether (x) Dimeric product (x) is : ...
Na dry ether (x) Dimeric product (x) is :
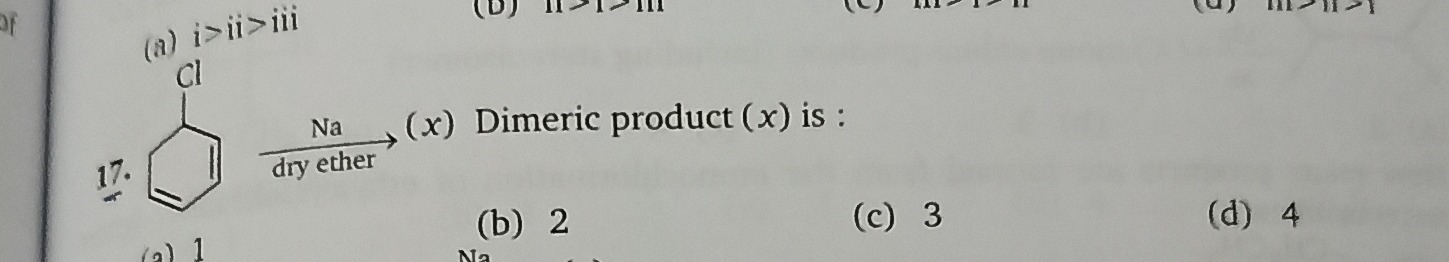
1
2
3
4
3
Solution
The reaction of 3-chlorocyclohex-1-ene with sodium in dry ether is a Wurtz-type coupling reaction involving an allylic halide. This reaction proceeds via the formation of a resonance-stabilized allylic radical.
1. Formation of the Allylic Radical:
The starting material is 3-chlorocyclohex-1-ene. The chlorine atom is at an allylic position (adjacent to a carbon-carbon double bond). When it reacts with sodium, the carbon-chlorine bond undergoes homolytic cleavage (or via an organosodium intermediate which then forms a radical), generating an allylic radical.
The initial radical (let's call it Radical A) is formed at C3:
.
C3
/ \
C2==C1
| |
C4 C6
\ /
C5
This radical is resonance stabilized. The double bond can shift, moving the radical character to C1. This gives the second resonance form (let's call it Radical B):
C3=C2
| \
C1(.)
| |
C6 C4
\ /
C5
Radical A has the double bond between C1 and C2, and the radical at C3. Radical B has the double bond between C2 and C3, and the radical at C1. These two radical forms are distinct because the positions of the double bond and the radical are different relative to the ring's overall structure and the numbering system.
2. Dimerization (Coupling) of Radicals:
Two such cyclohexenyl radicals can combine to form dimeric products. Since the radical character is delocalized over two different carbon atoms (C1 and C3), three distinct coupling products are possible:
-
Product 1: Coupling of two Radical A units (C3-C3 coupling)
Two units of Radical A combine by forming a bond between their C3 carbons.
C3-----C3' / \ / \ C2==C1 C1'==C2' | | | | C4 C6 C6' C4' \ / \ / C5 C5'This product has two cyclohexenyl rings joined at their 3-positions, and each ring retains the double bond between C1 and C2.
-
Product 2: Coupling of two Radical B units (C1-C1 coupling)
Two units of Radical B combine by forming a bond between their C1 carbons.
C3=C2 C3'=C2' | \ | \ C1-----C1' | | | | C6 C4 C6' C4' \ / \ / C5 C5'This product has two cyclohexenyl rings joined at their 1-positions, and each ring has the double bond between C2 and C3.
-
Product 3: Coupling of Radical A and Radical B (C1-C3 coupling)
One unit of Radical A combines with one unit of Radical B. The bond forms between the C3 of Radical A and the C1 of Radical B.
C3-----C1' / \ / \ C2==C1 C2'==C3' | | | | C4 C6 C6' C4' \ / \ / C5 C5'This product has one ring with a C1=C2 double bond (from Radical A) and the other ring with a C2'=C3' double bond (from Radical B), joined by a C3-C1' bond.
These three dimeric products are structurally distinct from each other.
Therefore, three different dimeric products are possible.
