Question
Question: Decreasing order of acidic strength of different (-OH) groups is:...
Decreasing order of acidic strength of different (-OH) groups is:
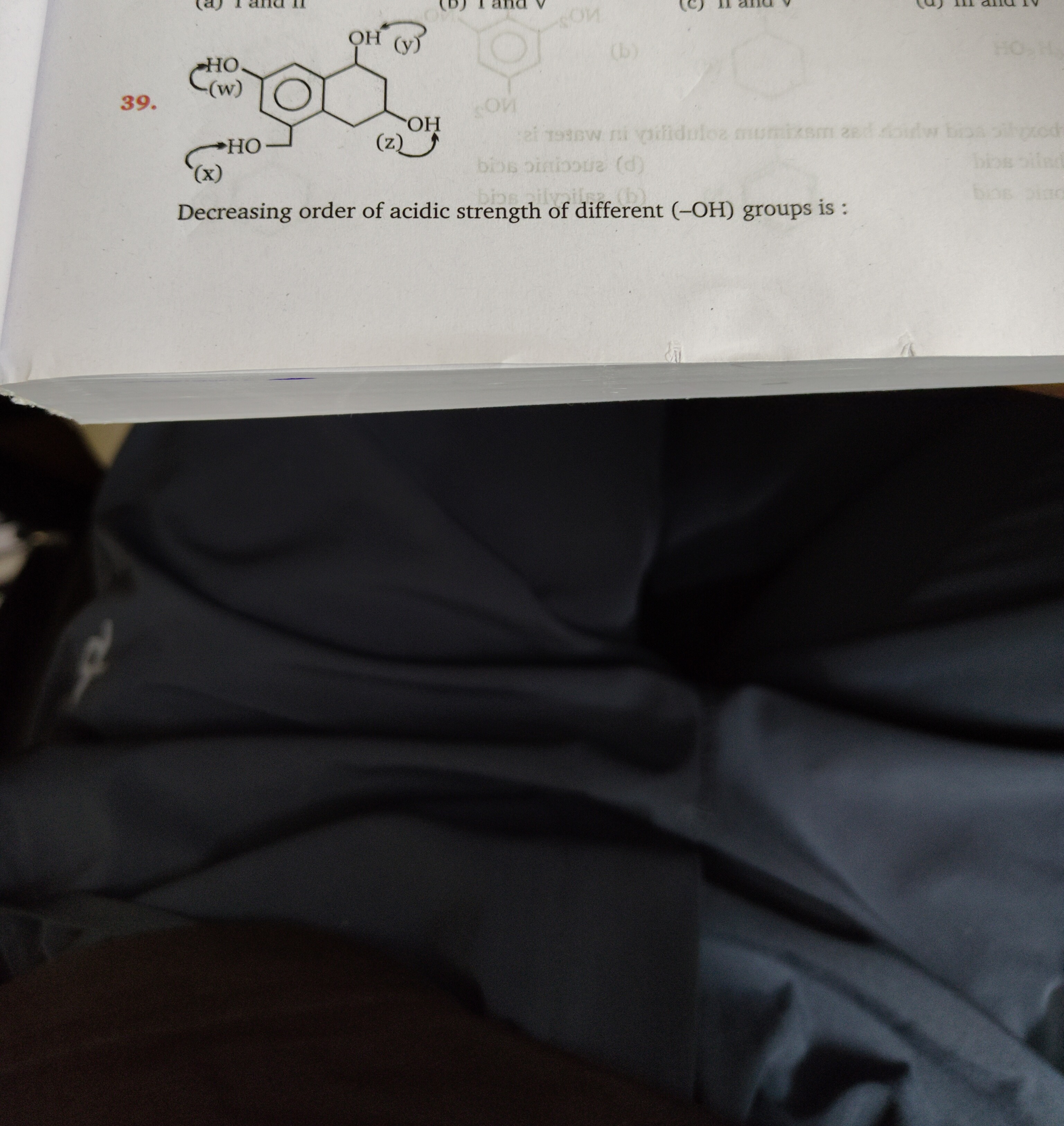
I and II
II and III
III and IV
I and IV
z > y > x > w
Solution
The acidic strength of a phenolic -OH group is determined by the stability of the phenoxide ion formed upon deprotonation. Factors that stabilize the phenoxide ion increase acidity. These include electron-withdrawing groups (EWGs) and resonance or inductive effects that delocalize the negative charge.
The molecule is 2,3,4,6-tetrahydroxybenzoic acid. Let's assign positions:
- -COOH group at C1.
- -OH(y) at C2 (ortho to -COOH).
- -OH(w) at C3 (meta to -COOH).
- -OH(x) at C4 (para to -COOH).
- -OH(z) at C6 (ortho to -COOH).
We analyze the acidity of each -OH group based on the substituents:
-
-OH(y) and -OH(z) (ortho to -COOH):
- The -COOH group is a strong electron-withdrawing group (EWG). Its inductive (-I) and resonance (-M) effects strongly stabilize the phenoxide ion formed at the ortho positions.
- These -OH groups can form intramolecular hydrogen bonds with the -COOH group, stabilizing the neutral molecule, which tends to decrease acidity. However, the phenoxide ion is a stronger base and can form an even stronger hydrogen bond with the -COOH group, stabilizing the phenoxide ion and increasing acidity.
- -OH(y) and -OH(z) are ortho to each other. The phenoxide ion formed at one ortho position can form a strong intramolecular hydrogen bond with the hydrogen of the adjacent -OH group, further stabilizing the phenoxide ion.
-
-OH(x) (para to -COOH):
- The -COOH group's resonance effect (-M) strongly stabilizes the phenoxide ion formed at the para position by delocalizing the negative charge. The inductive effect (-I) is weaker than at the ortho position.
-
-OH(w) (meta to -COOH):
- The -COOH group's inductive effect (-I) is weaker at the meta position. There is no direct resonance stabilization of the phenoxide ion by the -COOH group. Therefore, -OH(w) is expected to be the least acidic among the groups influenced by -COOH.
Relative Acidity Order: Based on the influence of the -COOH group, the general acidity order is ortho > para > meta. Thus, {y, z} > x > w.
Comparing -OH(y) and -OH(z): Both are ortho to -COOH and ortho to each other, benefiting from similar stabilizing effects. However, we must consider the influence of other -OH groups.
- -OH(y): It is para to -OH(x). The electron-donating resonance effect (+M) of -OH(x) destabilizes the phenoxide ion at C2.
- -OH(z): It is meta to -OH(x). The +M effect of -OH(x) does not directly destabilize the phenoxide ion at C6.
The destabilizing effect of the para -OH(x) group on the phenoxide at C2 makes -OH(y) less acidic than -OH(z). Therefore, -OH(z) is expected to be more acidic than -OH(y).
Considering other -OH groups: Phenolic -OH groups are electron-donating by resonance (+M), which generally destabilizes phenoxide ions and decreases acidity. However, they are electron-withdrawing by induction (-I), which stabilizes phenoxide ions and increases acidity. The net effect depends on the positions and interplay. In this complex molecule, the strong EWG effect of -COOH dominates. The ortho-ortho H-bonding between phenoxide and phenol is a significant stabilizing factor for both y and z.
The most acidic groups are those ortho to the strong EWG (-COOH), followed by the para group, and then the meta group. Among the ortho groups, the one less destabilized by other substituents is more acidic.
The decreasing order of acidic strength is z > y > x > w.
