Question
Question: A hydrogen-oxygen fuel cell operates under standard conditions. The standard half-cell reduction pot...
A hydrogen-oxygen fuel cell operates under standard conditions. The standard half-cell reduction potential for oxygen is E°(O₂/H₂O) = 1.20 V. For the complete reaction: 2H₂(g) + O₂(g) → 2H₂O(l) If 1 mole of O₂ is consumed in the fuel cell, the magnitude of the maximum electrical work obtained is x kJ. Find the value of x. (Nearest integer). (Given: 1 F = 96500 C mol⁻¹)
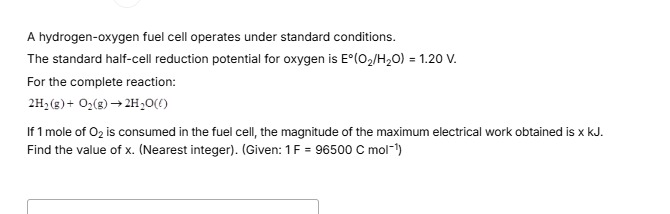
463
Solution
The maximum electrical work (Wmax) obtained from an electrochemical cell is given by the magnitude of the standard Gibbs free energy change, ∣ΔG∘∣, which is related to the cell potential (Ecell∘) by the equation: Wmax=∣ΔG∘∣=nFEcell∘ where:
- n is the number of moles of electrons transferred in the balanced reaction.
- F is Faraday's constant (96500 C mol⁻¹).
- Ecell∘ is the standard cell potential.
The given overall reaction is: 2H2(g)+O2(g)⟶2H2O(l)
From the half-reactions: Oxidation: 2H2(g)⟶4H+(aq)+4e− Reduction: O2(g)+4H+(aq)+4e−⟶2H2O(l) The number of moles of electrons transferred (n) is 4 for the consumption of 1 mole of O2.
The standard cell potential (Ecell∘) is calculated as: Ecell∘=Ecathode∘−Eanode∘ Given E∘(O2/H2O)=1.20 V (cathode). The standard reduction potential for the hydrogen electrode (anode) is E∘(H+/H2)=0.00 V. So, Ecell∘=1.20 V−0.00 V=1.20 V.
Now, calculate the maximum electrical work when 1 mole of O2 is consumed (n=4): Wmax=nFEcell∘ Wmax=(4 mol)×(96500 C mol−1)×(1.20 V) Wmax=463200 J
Convert Joules to kilojoules: Wmax=1000463200 kJ=463.2 kJ
The question asks for the value of x in kJ, rounded to the nearest integer. x=463.2 kJ. Rounding to the nearest integer gives x=463.
