Question
Question: A hydrocarbon of molecular formula \({{C}_{5}}{{H}_{10}}\) on monochlorination gives one product and...
A hydrocarbon of molecular formula C5H10 on monochlorination gives one product and on chlorination gives three products (excluding the stereoisomers). Identify the hydrocarbon.
Solution
It is an alicyclic hydrocarbon and a highly flammable compound. This compound is made by the cracking of cyclohexane in the presence of high temperature and pressure.
Complete step by step answer:
- This question is done basically by a hit and trial method.
- From the first part of the question which is the molecular formula, C5H10 comes under the general formula of CnH2n.
- From here we can get to know that the compound is either an alkene or a cyclic compound.
- So, the possible molecules are pentene and cyclopentane.
- From this point, we can look at the second part of the question, which says that the monochlorination of this compound should give only one product.
- In case of pentene (whether it is 1-pentene or 2-pentene), there are two α-hydrogens and so, on monochlorination, there will be two different products formed.
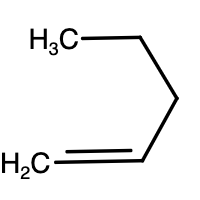
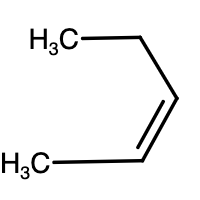
- Above given are the structures of pentene. The reaction is given as,
CH2=CHCH2CH2CH3+HCl→Cl−CH2−CH2CH2CH2CH3+CH3−Cl∣CH−CH2CH2CH3
-As we can see above, monochlorination of 1-pentene results in two different products. Similar reaction will take place for 2-pentene as well.
- So, we can now check the monochlorination product of cyclopentane.
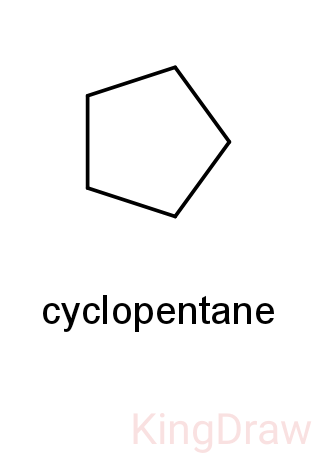
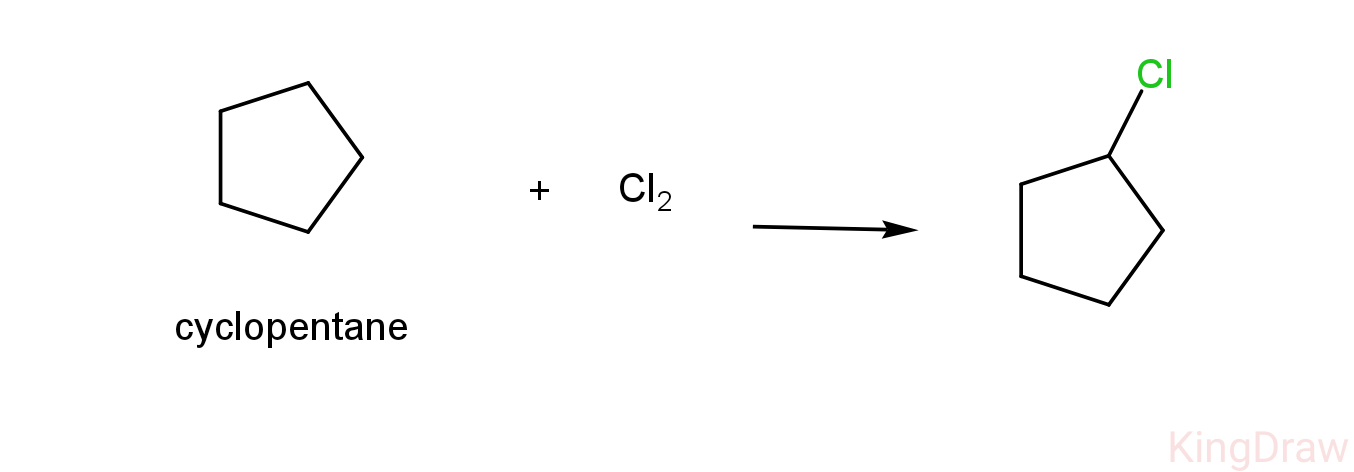
-As we can see above, cyclopentane reacts with chlorine to form only one product which is 1-chlorocyclopentane.
- Now, to confirm the compound, we can see the next part of the question which says that on dichlorination it should give three products
- Given below is the dichlorination of cyclopentane.
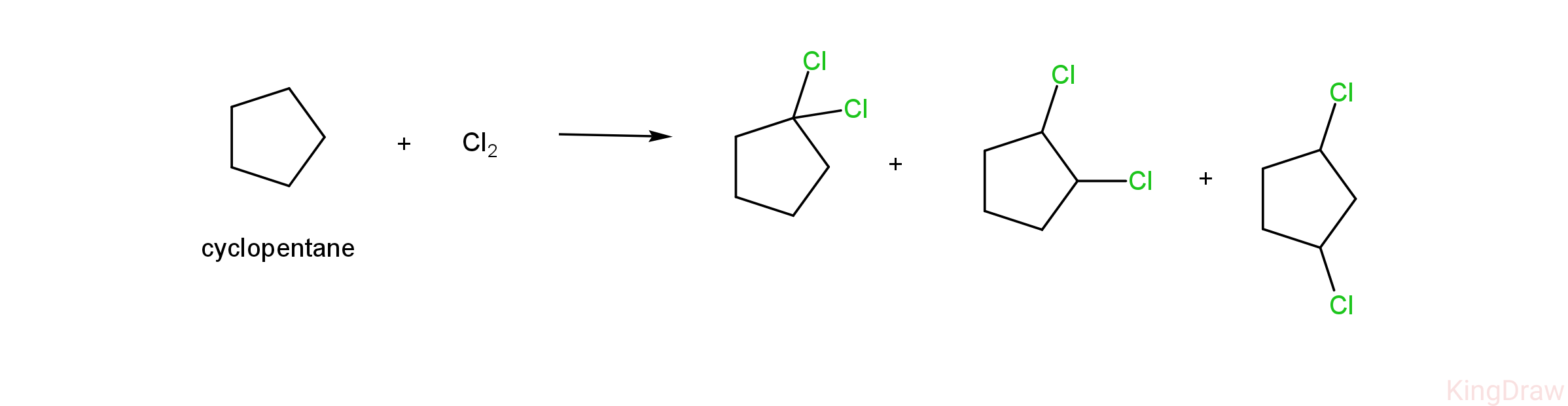
- As we can see, the dichlorination of cyclopentane results in the formation of three products, which are 1,1-dichlorocyclopentane, 1,2 -dichlorocyclopentane and 1,3-dichlorocyclopentane.
- Therefore , we can conclude that cyclopentane is the compound having the molecular formula of C5H10, on monochlorination giving one product (1-chloro cyclopentane) and on dichlorination gives three products (1,1-dichloro cyclopentane, 1,2 -dichlorocyclopentane and 1,3- dichlorocyclopentane)
- So, the answer is Cyclopentane.
Note: Monochlorination and dichlorination both the reaction proceeds under different sets of conditions. Monochlorination of cyclopentane occurs with Cl2 in the presence of light (hυ) whereas dichlorination of cyclopentene occurs when excess of Cl2 is provided in the presence of light (hυ).
