Question
Question: A hydrocarbon has the molar mass \[86\].The number of chain isomers possible for the compound is A...
A hydrocarbon has the molar mass 86.The number of chain isomers possible for the compound is
A.five
B.six
C.four
D.ten
Solution
From the given question we know that molar mass of a hydrocarbon is 86, we know that the general formula of alkanes is CnH2n+2 so we will assume that maximum number of carbon should be 6 and the remaining will be its hydrogen. We know that if the carbon chain will be more than 6 the molecular mass will be greater than 86.
Complete step by step answer:
As we know from the hint the hydrocarbon has maximum of 6 number of carbon so I am writing chemical formula for it which is C6H14
Checking with molecular formula 12×6′C′+14×1′H′=86
Hence satisfy given molecular formula according to question and next step is chain isomerism
Chain isomers are molecules with the same molecular formula, but different arrangements of the carbon structure.
Now I’m drawing possibilities of chain isomerism of C6H14
Firstly I’m taking simple structure with straight chain
CH3−CH2−CH2−CH2−CH2−CH3
Hexane
Now just adding CH3group on the second carbon chain which is different from above structure.
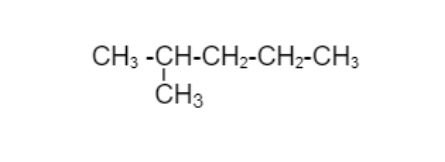
2 - Methylpentane
Adding two CH3 group on second carbon which is different from above two diagram
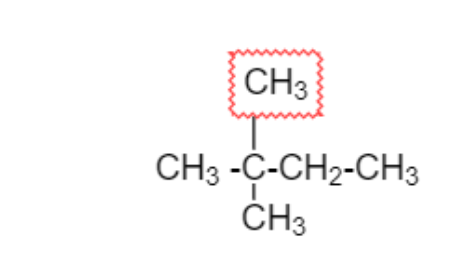
2,2−Dimethylbutane
Here in below diagram straight chain is CH3−CH2−CH2−CH3in this straight chain
I’m adding C2H5to second carbon and third carbon which is different from above
diagram
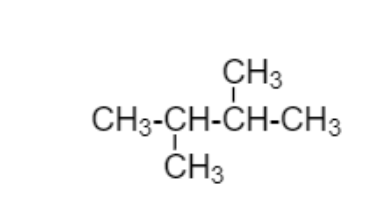
2,3−Dimethylbutane
In the below diagram straight chain is CH3−CH2−C−CH3AND I’m adding CH3on third carbon atom .
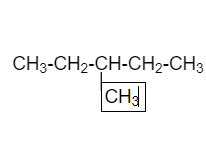
3−methylbutane
Shown images are the number of possible chain isomerism so they are 5isomerism structure.
Hence answer to this question is option ”A”.
Additional information:
A compound of hydrogen and carbon, like any of these which are the chief components of petroleum and gas
Chain isomerism
It is also referred to as skeletal isomerism.
The components of those isomers display differently branched structures.
Commonly, chain isomers differ within the branching of carbon
Scientists have tried to mathematically derive the amount of isomers of straight-chain organic molecules, called alkanes, but have discovered no simple relationships between isomer count and carbon content. However, computer programs that decompose alkane structures into manageable fragments give good results about alkanes:
Alkanes are chains of carbon (C) and hydrogen (H) atoms. For every n carbon atom there are (2n+2) hydrogen atoms. Alkanes originate principally from natural gas and crude oil. The carbon in alkanes forms chains that bind carbon to four other atoms through either C−C or C−H bonds. Straight (acyclic) alkanes don't form ring structures. The simplest alkane is methane (CH4). Alkanes with four or more carbon atoms can form structural isomers, and those with seven or more carbons can also form optical isomers.
Note: The above given structures are chain isomers to each other and if we want to draw any structure they will look the same like this five structure. It's mathematically impossible to calculate the number of isomers of alkanes, but computer programs use an algorithm to work it out.
