Question
Question: A hydrocarbon \({{C}_{6}}{{H}_{10}}\) absorbs only one molecule of \({{H}_{2}}\) on catalytic hydrog...
A hydrocarbon C6H10 absorbs only one molecule of H2 on catalytic hydrogenation. On ozonolysis, the hydrocarbon yields CHO(CH2)4CHO. The hydrocarbon is:
(A) Cyclohexene
(B) 1,5-hexadiene
(C) 1,3-cyclohexadiene
(D) 1-methylcyclopentene
Solution
From the molecular formula C6H10 , we can say that the hydrocarbon contains one double bond in its structure. On ozonolysis C6H10 giving a symmetrical dialdehyde ( CHO(CH2)4CHO ) as the product means the alkene should be symmetrical in nature.
Complete step by step solution:
-In the question, it is given that C6H10 absorbs one mole of hydrogen gas on catalytic hydrogenation, means the given hydrocarbon has unsaturation (one double bond) in its structure.
-It is given that on ozonolysis C6H10 yields CHO(CH2)4CHO as the product.
-Coming to the given options, option A, Cyclohexene.
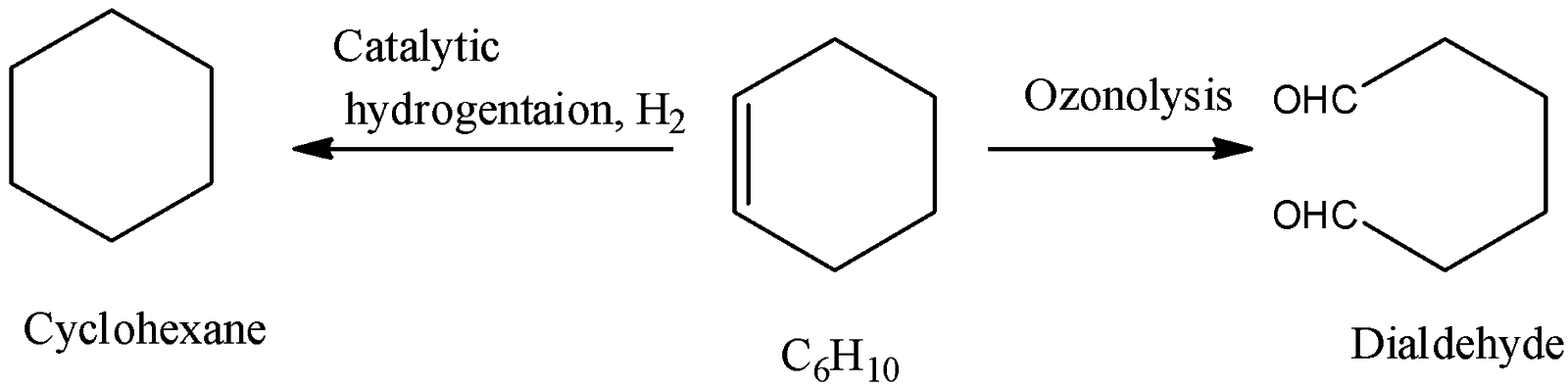
-Cyclohexene ( C6H10 ) on reaction with one mole of hydrogen and forms cyclohexane and on ozonolysis forms symmetrical dialdehyde as the product. So, option A is correct.
-Coming to option B, 1,5-hexadiene.
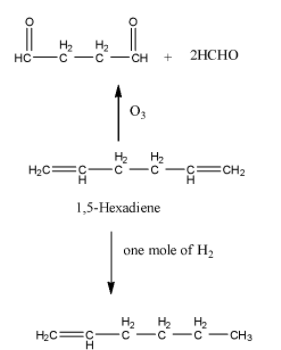
-Option B is wrong, because in the question it is given that on ozonolysis the hydrocarbon should give a single product. But on ozonolysis 1,5-Hexadiene gives two products. So, option B is wrong.
-Coming to option C, 1,3-cyclohexadiene.
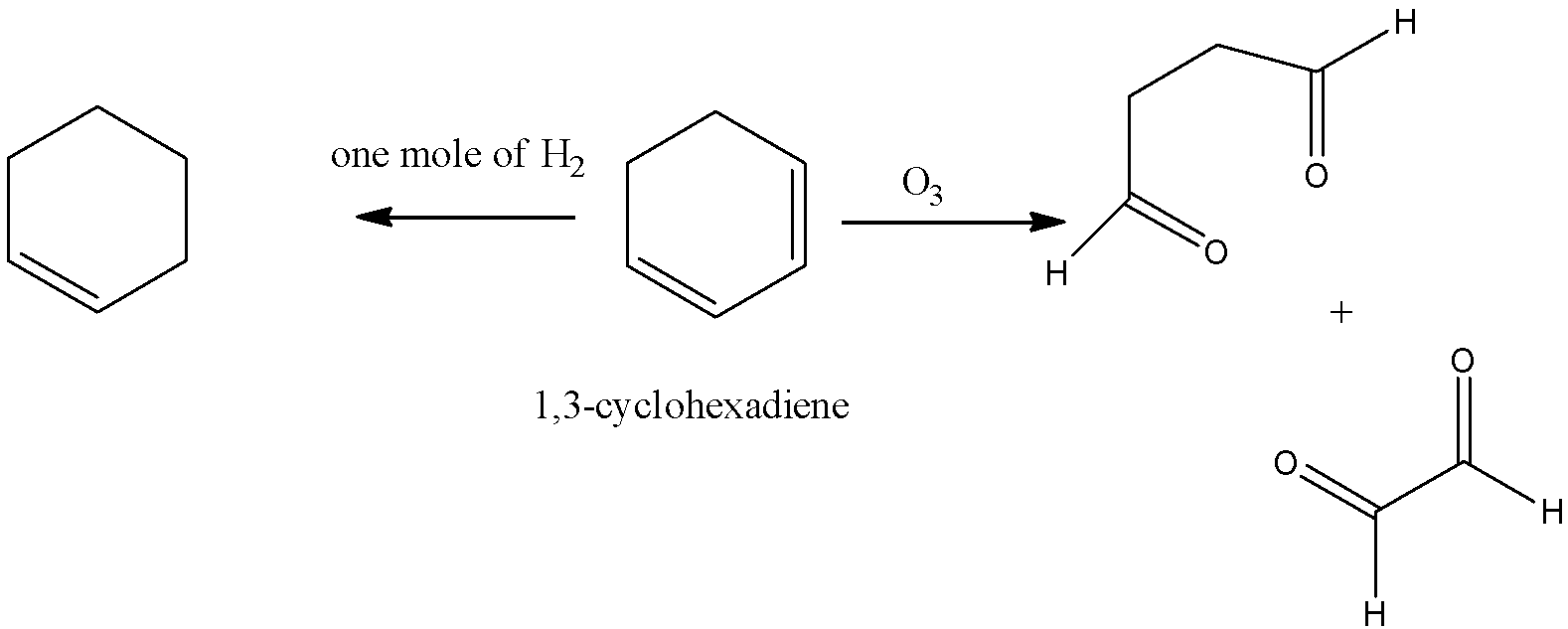
-In the question it is given that on ozonolysis the hydrocarbon should give only one product. But 1,3-cyclohexadiene is giving two products on ozonolysis. So, option C is wrong.
-Coming to option D, 1-methylcyclopentene.
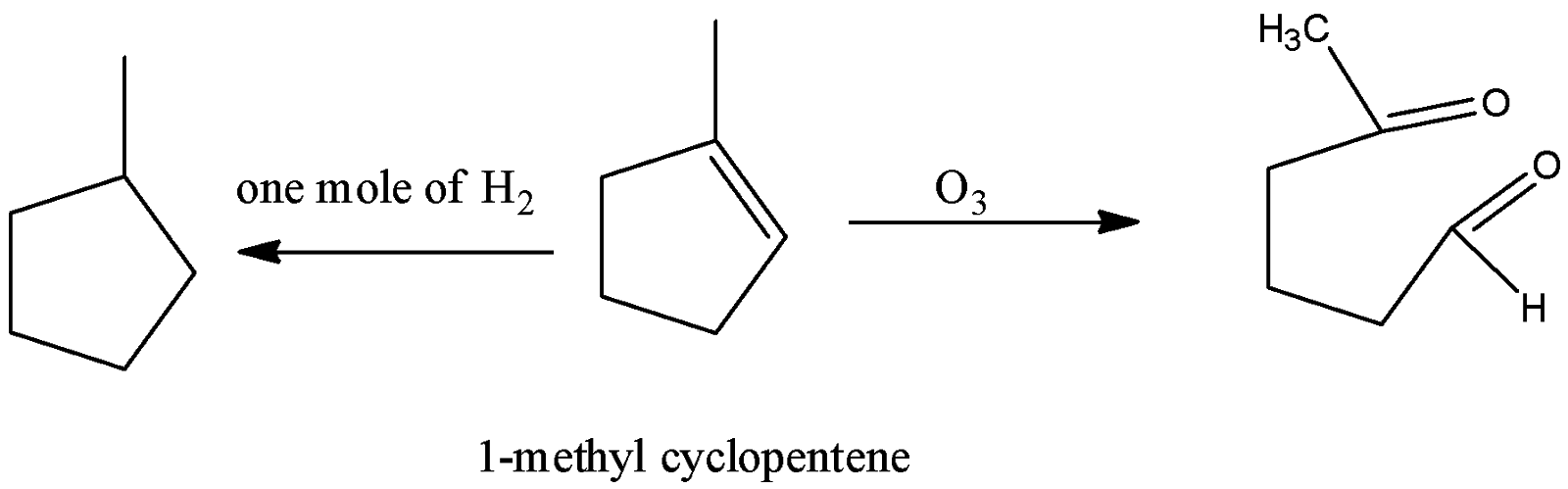
-In the question it is given that on ozonolysis the hydrocarbon should give a symmetrical dialdehyde.. But 1- methyl cyclopentene is giving an unsymmetrical product on ozonolysis. So, option D is wrong.
-Therefore, cyclohexene is going to match with the desired properties mentioned in the question.
So, the correct option is (A).
Note: Catalytic hydrogenation and ozonolysis are the best tests to identify the presence of unsaturation in the hydrocarbons. On hydrogenation alkenes gives alkanes and on ozonolysis alkenes gives respective carbonyl compounds as the products.
