Question
Question: A hydrocarbon A (V.D = 36) forms only one monochloro substitution product. A will be (A) isopentan...
A hydrocarbon A (V.D = 36) forms only one monochloro substitution product. A will be
(A) isopentane
(C) cyclohexane
(D) methyl cyclohexane
Solution
We can use the vapour density given to find the hydrocarbon A. Twice of the vapour density of the hydrocarbon A will give the molecular weight of the compound. On knowing the molecular mass, we can easily find the hydrocarbon A.
Complete Solution :
Vapour density of the hydrocarbon is given which is 36.
Molecular weight of the hydrocarbon =2×36=72
Calculation of the molecular weight suggests that it is alkane.
The general formula of alkane is CnH2n+2.
Hydrocarbon A =CnH2n+2=12n+2n+2=72
The hydrocarbon formed is C5H12.
The hydrocarbon A on chlorination will give monochloro substituted product i.e. 1-Chloro-2,2-dimethylpropane.
The hydrocarbon A with the formula C5H12 is Neopentane or 2,2-Dimethylpropane.
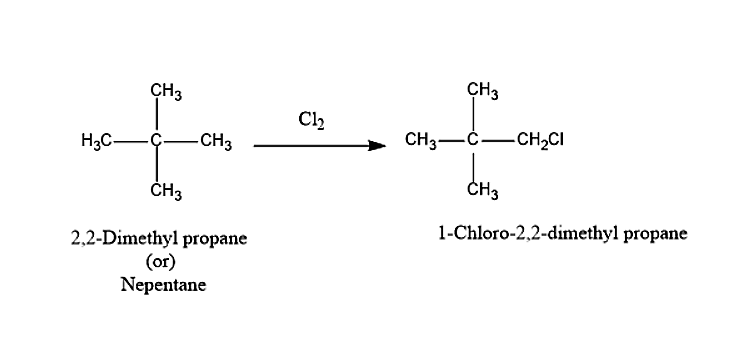
So, the correct answer is “Option B”. neo-pentane.
Additional information:
Let us see some of the general characteristics of Neopentane.
- Neopentane is also known as 2,2-Dimethylpropane.
- It is similar to butane physically.
- It is highly flammable gas.
- Neopentane is low molecular weight alkane.
- It is a component of petroleum fuel mixture.
- It is less toxic.
So, the correct answer is “Option B”.
Note: The molecular weight that we have found is different from the molecular mass. The molecular weight is also known as the molar mass. The molecular weight is the weight or mass of one mole of the substance.
