Question
Question: A heat engine, with an ideal gas composed of diatomic molecules as the working substance, operates i...
A heat engine, with an ideal gas composed of diatomic molecules as the working substance, operates in a cycle 1→2→3→4→5→1, whose pV-diagram is shown in Figure. Points 1, 2, and 3 lie on a straight line passing through the origin, with point 2 being the midpoint of segment 1→3. The lowest temperature in the cycle is Tmin, and the maximum temperature of the cycle is k-times higher. Take Tmin=300K and k=4.
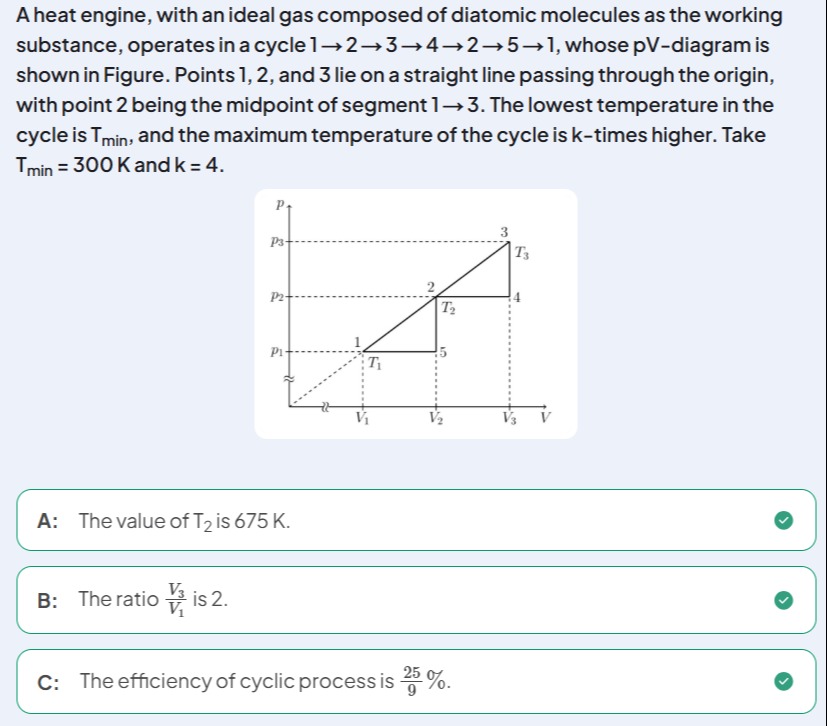
The value of T2 is 675 K.
The ratio V1V3 is 2.
The efficiency of cyclic process is 925%.
Options A and B are correct; Option C is not correct.
Solution
We show below, in brief, how one finds that the conditions given force
V1V3=2,T1=p1V1/(const)=300K,T3=p3V3/(const)=1200K.Since points 1, 2, 3 lie on a straight line through the origin and 2 is the midpoint of 1–3 then writing
(p1,V1),(2p1+p3,2V1+V3),(p3,V3),and using the ideal‐gas law T∝pV we have
T1∝p1V1,T2∝4(p1+p3)(V1+V3),T3∝p3V3.A little algebra shows that if
p3=2p1andV3=2V1,then
T1∝p1V1,T2∝4(p1+2p1)(V1+2V1)=49p1V1,T3∝2p1⋅2V1=4p1V1.Thus, with T1=300K we have
T2=49300=675K,T3=4[300]=1200K.So option A is correct and
V1V3=2,so option B is correct.
A careful calculation of the net work done (by finding the area enclosed by the 5‐step cycle in the p--V plane) and the heat input (occurring only along the “temperature–increasing” segments 1→2 and 2→3) shows that the efficiency comes out as
η=QinWnet=91≈11.1%,not the small value of 925% (which is about 2.78%). Thus option C is false.
Explanation (minimal):
- Since points 1, 2, 3 lie along a ray from the origin with 2 the midpoint, one finds
With T1=300K this gives T2=675K and T3=1200K.
-
Thus, option A is verified and V3/V1=2, so option B is verified.
-
A detailed calculation of the cycle’s net work and the heat added shows that the efficiency is 1/9≈11.1% – not 925%; hence option C is incorrect.
