Question
Question: A group of \(13\) elements if added in small amounts to \(Ge\) , then the type of semiconductor form...
A group of 13 elements if added in small amounts to Ge , then the type of semiconductor formed is :
A . n-type semiconductor
B . p-type semiconductor
C . super semiconductor
D . both A and B
Solution
Hint : We know that when a small amount of foreign impurity is added to a crystal then this process is called doping. Doping increases electrical conductivity of the crystal. We have learned about two types of semiconductor i.e. extrinsic semiconductors and intrinsic semiconductors. The pure semiconductor which does not go through the process of doping is called intrinsic semiconductor and an extrinsic semiconductor is one that has been doped.
Complete answer:
- We know about two types of doping agents which result in two types of semiconductor .
- When the donor is an electron donor atom then it is called n-type semiconductor while when the dopant is electron acceptor it is called p-type semiconductor. The majority charge carriers in p-type semiconductors and n-type semiconductors are holes and electrons respectively. Extrinsic semiconductors are used as the components of many semiconductor devices.
- Group 13 elements in the periodic table are the boron family. The members of group 13 are boron, aluminium, gallium ,indium, thallium. Elements of this group 13 have three valence electrons. So these elements will give electrons when we add them in pure semiconductor. Germanium (Ge) has a similar nature like silicon and is used to make semiconductor devices. So a group of 13 elements if added in small amounts to Ge , then the type of semiconductor formed is p-type semiconductor. Hence option B is correct
.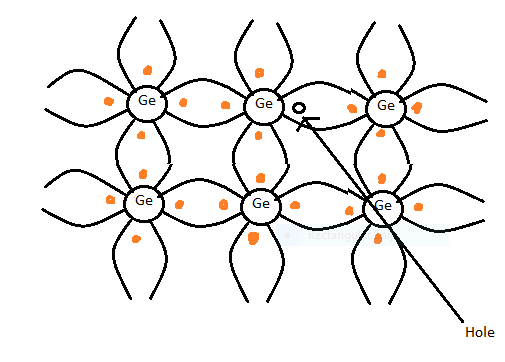
Note : Here we have approached this problem with the help of concept that when trivalent impurity atom is added to germanium it becomes p-type semiconductor so here option B is correct that is p-type semiconductor .In the above diagram we can see that there is bonding between germanium and trivalent impurity atoms.
