Question
Question: A group having ‘ –A=B’ type structure in which electronegativity of B is greater than A which is att...
A group having ‘ –A=B’ type structure in which electronegativity of B is greater than A which is attached to benzene ring the E+ attack mostly at:
(a)- m – position
(b)- o – position
(c)- p – position
(d)- All
Solution
By the type of compound attached to the benzene ring we can predict where the attack of the compound will take place. There are two types of attacks, i.e., electrophile attack and nucleophile attack. For electrophile attack, if the compound increases the electron density on the benzene ring then it will attack ortho and para position, and if the compound decreases the electron density on the benzene ring it will attack meta position.
Complete answer:
Benzene is an aromatic compound of six carbon atoms. The structure of benzene is given below:
When there is the addition of any molecule or compound to the benzene ring, it will be either a nucleophile or an electrophile. There are two types of attacks, i.e., electrophile attack and nucleophile attack. On the aromatic ring, mostly electrophile attack.
For electrophile attack, if the compound increases the electron density on the benzene ring then it will attack ortho and para position, and if the compound decreases the electron density on the benzene ring it will attack meta position.
Given the group attached to the benzene ring is ‘ –A=B'. Since B is more electronegative than A. There will be a negative charge on B and a positive charge on A. It is shown below:
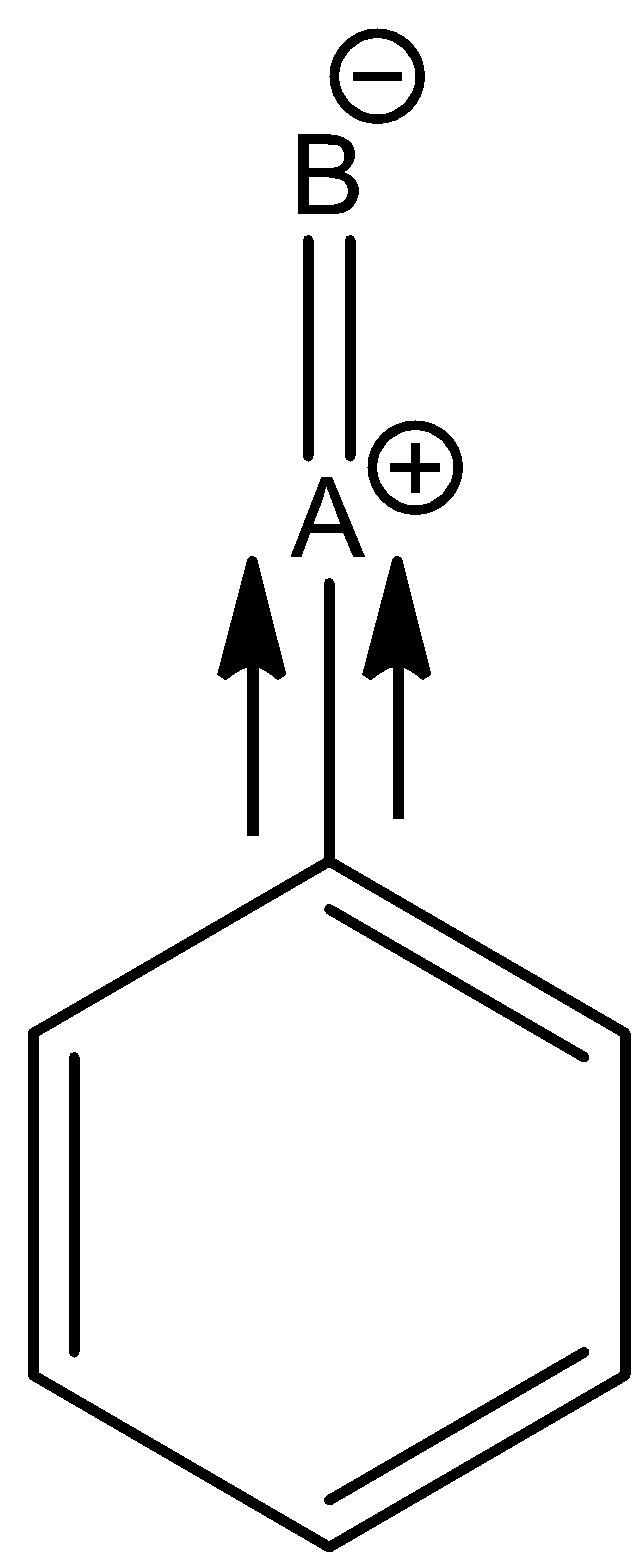
Due to the positive charge on A, it will attract electrons from the ring and it will decrease the electron density of the ring. So, it will attack the meta–position.
Therefore, the correct answer is an option (a)- m – position.
Note:
If the group increases the electron density of the ring, then it will attack either ortho or para position, but the major product will be a compound having a para-position substitute.
