Question
Question: A Grignard reagent reacts with water to give: (a)- Ether (b)- Alkane (c)- Amine (d)- Alcohol...
A Grignard reagent reacts with water to give:
(a)- Ether
(b)- Alkane
(c)- Amine
(d)- Alcohol
Solution
The Grignard reagent is a compound that comes under the organometallic compounds because there is carbon element as well as metal also present. The metal part is MgX, X is any halogen atom. In reaction with water, the MgX will react with the hydroxyl part of the water.
Complete answer:
When any compound reacts with water, we can say that a hydrolysis reaction occurs. The given compound in the question is the Grignard reagent.
We know that Grignard reagent is a compound that comes under the organometallic compounds because there is carbon element as well as metal also present. The general representation of the Grignard reagent is RMgX, where R is alkyl or aryl group and X is halogen like chlorine, bromine, or iodine.
In RMgX, the positive part of the cationic part is MgX, while the negative part or anionic part is the alkyl or aryl group. When the Grignard reagent reacts with water then the positive part of water, i.e., a hydrogen ion will react with the negative part of the Grignard reagent while the negative part of water, i.e., hydroxyl group will react with the positive part of the Grignard reagent. A general reaction is given below:
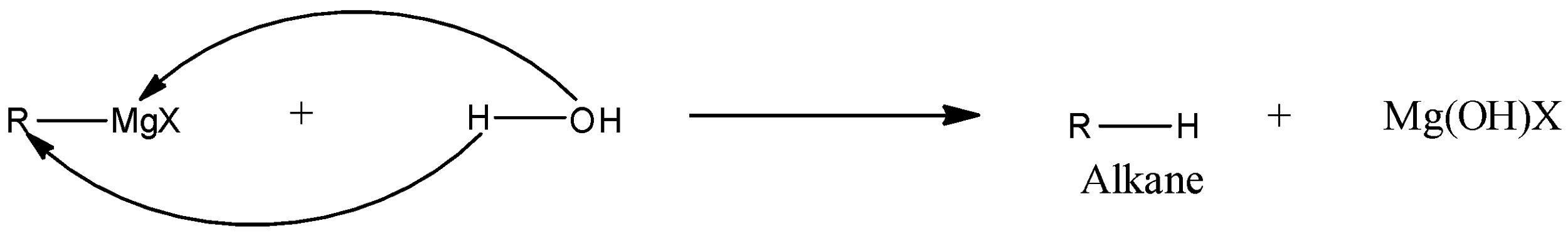
From the reaction, we can see that there is the formation of alkane.
Therefore, the correct answer is an option (b)- Alkane.
Note:
For example, you take CH3MgBr as the Grignard reagent and react it with water then it will form methane (CH4). In the Grignard reagent, we cannot take fluorine as the halogen, only Cl, Br, or I can form Grignard reagent.
