Question
Question: A graph regarding photoelectric effect is shown between the maximum kinetic energy of electrons and ...
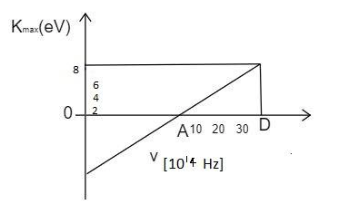
A graph regarding photoelectric effect is shown between the maximum kinetic energy of electrons and the frequency of the incident light . On the basis of the data as shown in the graph, calculate the work function.
(A) 4eV
(B) 2eV
(C) 4.2eV
(D) 2.5eV
Solution
Find the threshold frequency from the graph. Use the formula of the Planck’s constant and substitute the known parameters to find the value of the Planck’s constant. Substitute this value and the threshold frequency in the formula of the work function formula, to find the value of the work function.
Useful formula:
(1) The formula of the Planck’s constant is given by
h=ΔvΔKmax
Where h is the Planck’s constant, ΔKmax is the change in the kinetic energy and Δv is the change in the frequency
(2) The formula of the work function is given as
w=hv0
Where w is the work function, v0 is the threshold frequency.
Complete step by step solution:
Analyze the graph completely,
From the graph, it is clear that the threshold frequency is v0=10×1014Hz.
Using the formula of the Planck’s constant, we get
h=ΔvΔKmax
Substituting the known values and converting the unit of the electron volts into Joules by multiplying it with 1.6×10−19 ,
h=(30−10)×10148×1.6×10−19
By simplifying the above equation, we get
h=6.4×10−34J
In order to calculate the work function, use the formula of the work function,
w=hv0
Substituting the values of the Planck's constant and the threshold frequency, we get
w=6.4×10−34×10×1014
By performing the basic arithmetic operation, we get
w=4eV
Hence the work function from the given graph is 4eV .
Thus the option (A) is correct.
Note: The unit conversion from the electron volt to the Joules is made by multiplying it with 1.6×10−19 . The frequency of the 10×1014Hz is the minimum frequency that produces the photoelectric effect. This part lies above the horizontal axis of the graph.
