Question
Question: A graph between $log t_{1/2}$ and log a, a being initial concentration of A in reaction (A → Product...
A graph between logt1/2 and log a, a being initial concentration of A in reaction (A → Products) is given below
Select correct statement(s) based on it.
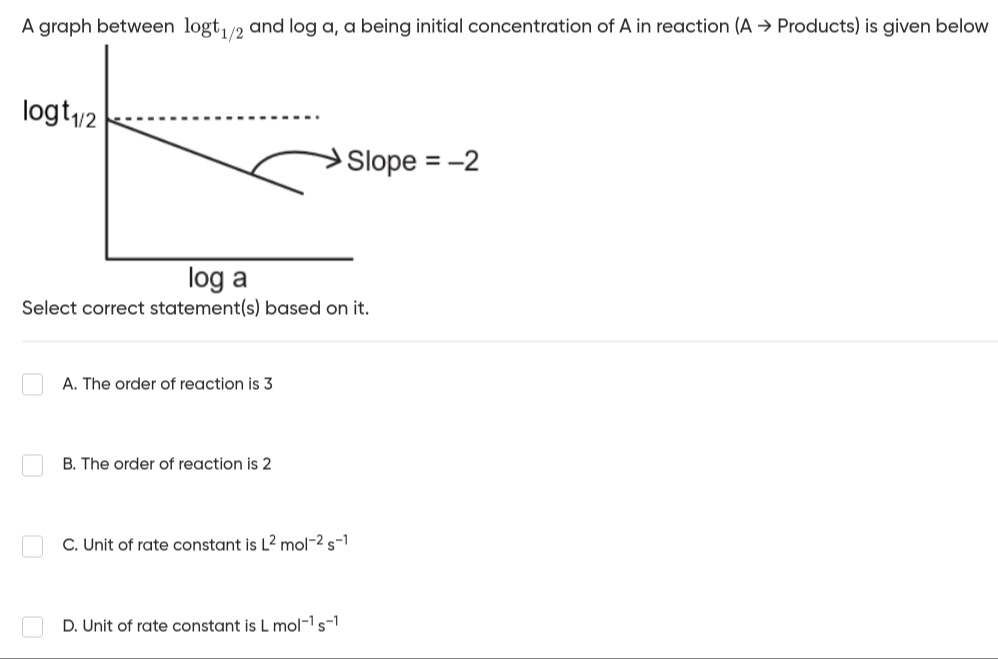
The order of reaction is 3
The order of reaction is 2
Unit of rate constant is L2mol−2s−1
Unit of rate constant is Lmol−1s−1
A, C
Solution
The half-life (t1/2) for an n-th order reaction (where n=1) is related to the initial concentration (a) by the equation: t1/2=k(n−1)1an−11=k(n−1)1a1−n
Taking the logarithm of both sides: logt1/2=log(k(n−1)1)+(1−n)loga
This equation is in the form of a straight line, Y=C+mX, where: Y=logt1/2 X=loga The slope of the graph (m) is (1−n).
From the given graph, the slope is -2. So, we can write: 1−n=−2 n=1−(−2) n=1+2 n=3
Therefore, the order of the reaction is 3.
Now, let's determine the unit of the rate constant (k) for a 3rd order reaction. The general unit of the rate constant for an n-th order reaction is given by: Unit of k=(concentration)1−n(time)−1 Using concentration in mol L−1 and time in seconds (s): Unit of k=(mol L−1)1−ns−1
For a 3rd order reaction (n=3): Unit of k=(mol L−1)1−3s−1 Unit of k=(mol L−1)−2s−1 Unit of k=mol−2L2s−1 This can also be written as L2mol−2s−1.
Therefore, statement C is correct.
