Question
Physics Question on Thermodynamics
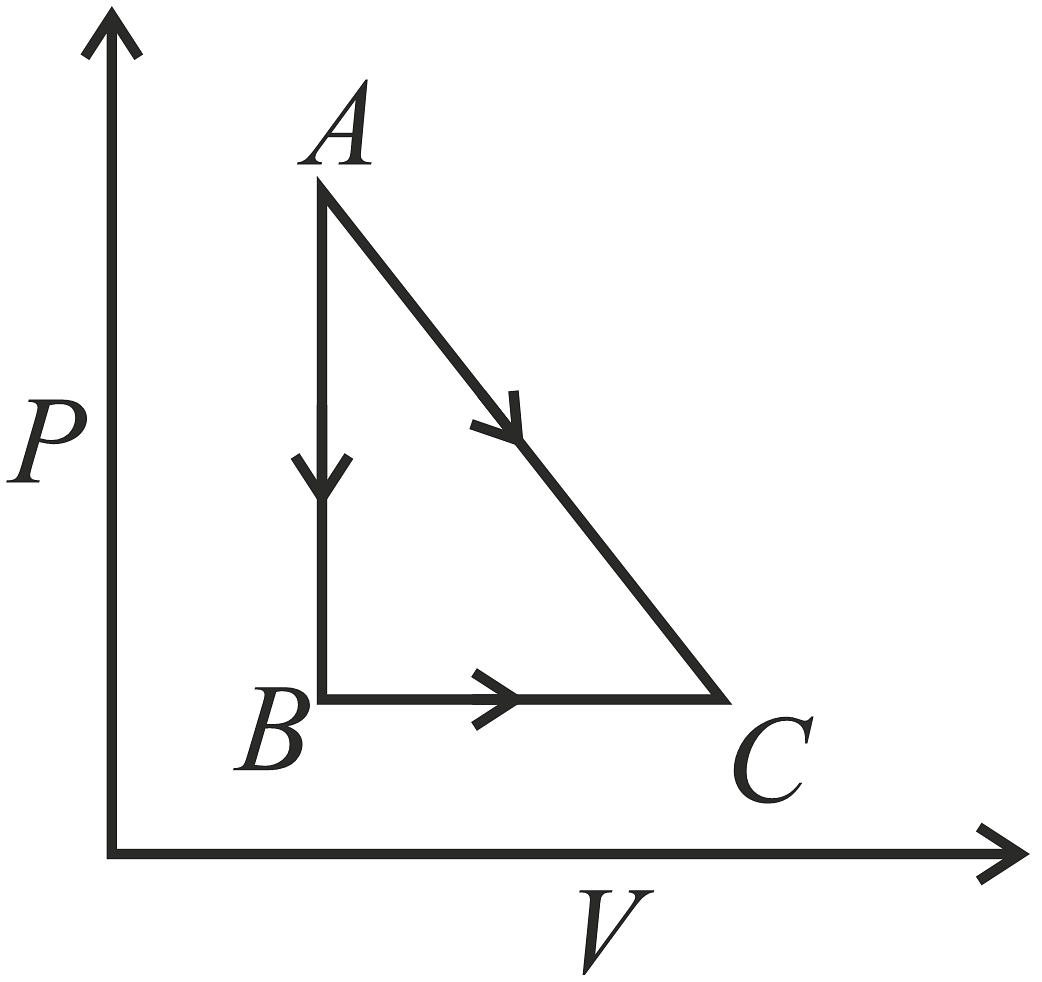
A given quantity of gas is taken from A to C in two ways; a) directly from A→C along a straight line and b) in two steps, from A→B and then from B→C.Work done and heat absorbed along the direct path A→C are 200J and 280J respectively. If the work done along A→B→C is 80J, then heat absorbed along the path is,
80J
0
160J
120J
160J
Solution
While moving along path AC, the change in internal energy (dU) is determined by the difference between heat (dQ) added to the system and work (dW) done by the system. In this case, dU =dQ − dW =300−200=100J. This illustrates that internal energy is a point function, which means it remains consistent across all paths.
Along path ABC, the heat (dQ) is the sum of the change in internal energy (dU) and the work (dW) done on the system.
The correct option is(C) 160J
