Question
Question: A given nitrogen containing aromatic compound A reacts with \[{\text{Sn/HCl}}\], followed by \[{\tex...
A given nitrogen containing aromatic compound A reacts with Sn/HCl, followed by HNO2 to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with molecular formula C12H10N2O. The structure of compound A is:
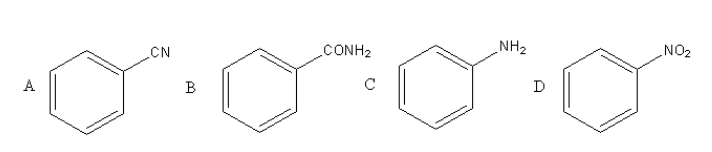
Solution
Sn/HCl will reduce the compound A. Nitric acid HNO2 cause the formation of diazonium ions. The diazonium ion will give the substitution reaction with phenol. The formed product will be a diazo compound.
Complete step by step solution:
Sn/HCl is a reducing agent, so it will reduce the compound A. HNO2 is known as nitric acid. The nitric acid is used for nitration. Nitric acid forms the diazonium ion with the amine functional group.
Amine forms by the reduction of the nitro functional group. So, compound A should be a nitro compound.
Compound A has a cyanide functional group so, option (A) is incorrect.
Compound B has an amide functional group so, option (B) is incorrect.
Compound C has an amine functional group so, option (C) is incorrect.
Compound D has a nitro functional group so, option (D) is correct.
The reaction of nitrobenzene with Sn/HCl and then with HNO2 is as follows:
The nitrobenzene gets reduced in presence of Sn/HCl and forms aniline. Aniline reacts with nitric acid and forms diazonium ion.
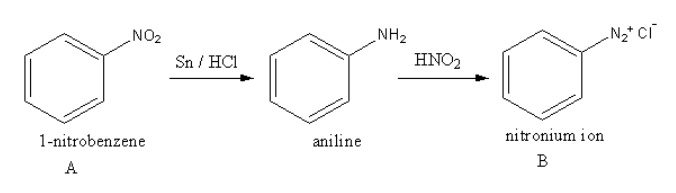
So, the unstable compound B is nitronium ion.
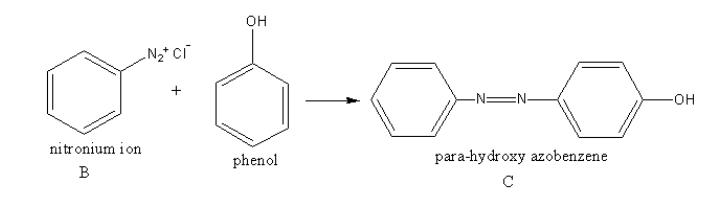
The compound B reacts with phenol to give para-hydroxy azobenzene.
The reaction of compound B with phenol is as follows:
So, the compound A is 1−nitrobenzene.
Therefore, option (D) is correct.
Note: The Sn + HCl is a reducing agent. The mixture of NaNO2 + HCl at high temperature is used for the preparation of nitric acid which gives diazonium chloride. The diazonium chloride gives an electrophile. The electrophile attacks on the para position of the phenol and replaces the proton forming the final product.
