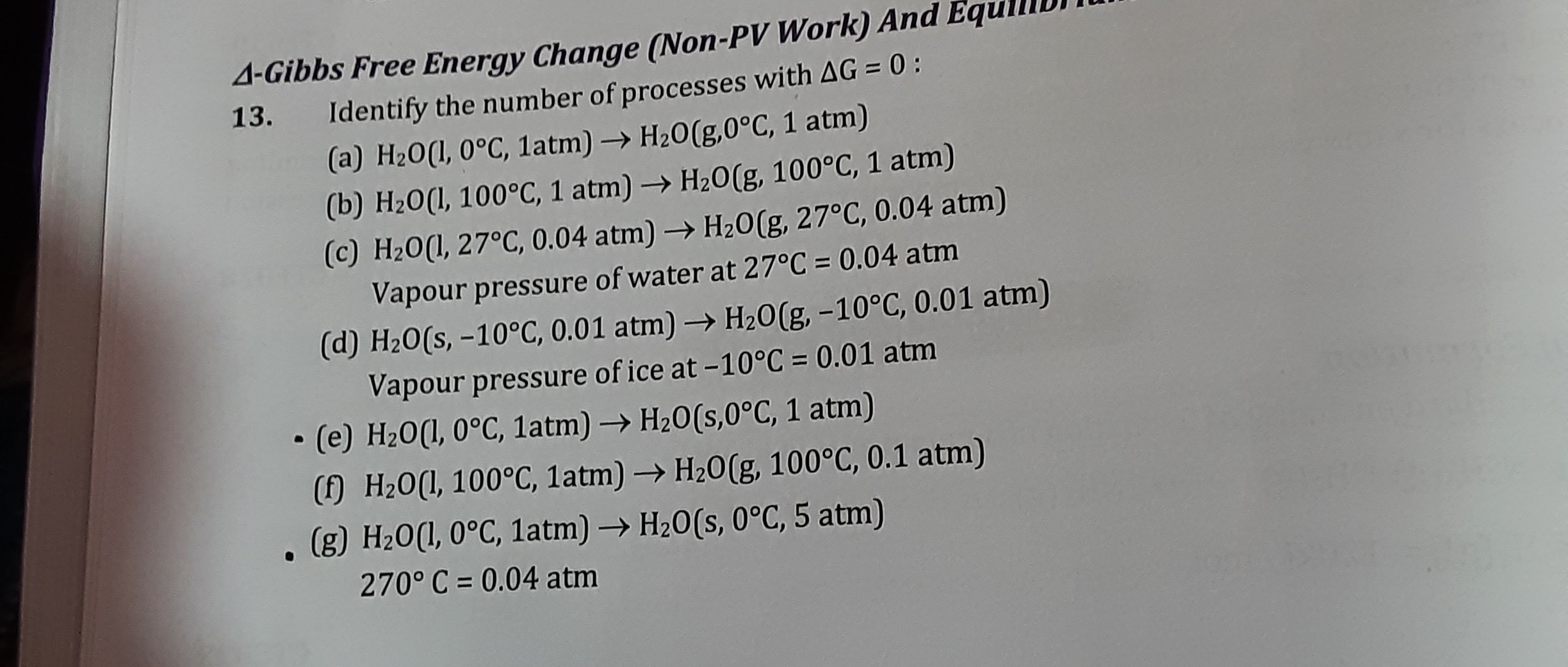
Question
Question: Identify the number of processes with $\Delta$G = 0 :...
Identify the number of processes with ΔG = 0 :
4
Solution
For a process occurring at constant temperature and pressure, ΔG=0 signifies that the system is at equilibrium. For a phase transition, this means the two phases are in equilibrium under the specified conditions of temperature and pressure.
Let's analyze each process:
-
(a) H2O(l, 0°C, 1atm) → H2O(g,0°C, 1 atm)
At 0°C, the equilibrium vapor pressure of water is approximately 0.006 atm. Since the given pressure is 1 atm, which is much higher than the equilibrium vapor pressure, liquid water is stable at these conditions, and it is not in equilibrium with water vapor at 1 atm. Therefore, ΔG=0. -
(b) H2O(l, 100°C, 1 atm) → H2O(g, 100°C, 1 atm)
100°C is the normal boiling point of water. At this temperature and 1 atm pressure, liquid water and water vapor are in equilibrium. Therefore, ΔG=0. -
(c) H2O(l, 27°C, 0.04 atm) → H2O(g, 27°C, 0.04 atm)
The problem states that the vapor pressure of water at 27°C is 0.04 atm. This means at 27°C, liquid water is in equilibrium with its vapor when the vapor pressure is 0.04 atm. The process describes this exact equilibrium condition. Therefore, ΔG=0. -
(d) H2O(s, -10°C, 0.01 atm) → H2O(g, -10°C, 0.01 atm)
The problem states that the vapor pressure of ice at -10°C is 0.01 atm. This refers to the sublimation pressure of ice. At -10°C, solid ice is in equilibrium with water vapor when the vapor pressure is 0.01 atm. The process describes this exact equilibrium condition. Therefore, ΔG=0. -
(e) H2O(l, 0°C, 1atm) → H2O(s,0°C, 1 atm)
0°C is the normal freezing (or melting) point of water. At this temperature and 1 atm pressure, liquid water and solid ice are in equilibrium. Therefore, ΔG=0. -
(f) H2O(l, 100°C, 1atm) → H2O(g, 100°C, 0.1 atm)
At 100°C, the equilibrium vapor pressure of water is 1 atm. The process describes liquid water at 100°C transforming into gaseous water at 0.1 atm. This is not an equilibrium condition for the phase transition. If liquid water at 100°C is exposed to a pressure of 0.1 atm, it will spontaneously boil. Therefore, ΔG=0. -
(g) H2O(l, 0°C, 1atm) → H2O(s, 0°C, 5 atm)
The normal freezing point of water is 0°C at 1 atm. For water, increasing pressure lowers the freezing point (because ice is less dense than liquid water). Therefore, at 0°C and a higher pressure of 5 atm, water would exist in the liquid phase. The system is not at equilibrium for freezing. Therefore, ΔG=0.
The processes for which ΔG=0 are (b), (c), (d), and (e). The number of such processes is 4.
