Question
Question: A gaseous compound is heated at constant pressure. The figure above shows the variation of volume as...
A gaseous compound is heated at constant pressure. The figure above shows the variation of volume as the temperature is increased.
At first, the volume increases in a steady fashion, as predicted by Charles' law. Then, all of a sudden, at Point A, the volume starts to increase at a more rapid rate.
Which of the choices is a possible explanation for this behaviour?
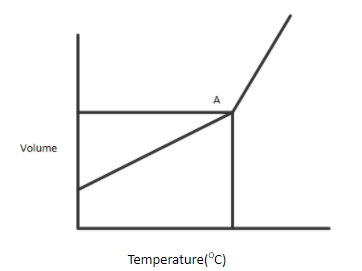
A.Gases cease to behave ideally at elevated temperature
B.The molecules of gas has begun to dissociate
C.Point A is the critical point for this substance
D.A change of state is occurring
Solution
Gas molecules have a finite volume and each other's attracting forces are experienced. When temperature is increased, normally there is an increase in kinetic energy and the volume increases at a normal rate. Here by observing the graph we can understand that there is a point above which an abnormal increase in volume is exhibited by the gas.
Complete answer:
Let us analyse each point one by one;
A.Real gases tend to behave like ideal gases at relatively high temperatures and low pressure. However, this cannot lead to an abnormal increase in the volume from a point as real gases occupy more volume than ideal gases at a given pressure. Hence, this option is not correct.
B.As temperature increases, the dissociation constant increases which in turn lead to the decrease in the degree of dissociation of gas molecules. Hence, this is not the correct option.
C.At point A their volume is unexpectedly increased by a slight rise in temperature because of the formation of supercritical fluid. Point A is also known as the critical point. The critical point is the temperature and pressure at which the difference can no longer be made between liquid and gas. The molecules in a closed container are assumed to vaporise at such an accelerated rate at the critical point that the density of liquid and vapour is equal and thus forms a supercritical fluid. The resulting temperature and pressure are defined as the critical temperature and critical pressure. If the pressure on a gas (vapour) is elevated at a temperature below the critical temperature, the liquid-vapor equilibrium boundary will finally be reached and the vapour will condense to provide a liquid. At critical temperature, the kinetic energy of the molecules will be high and hence, this results in an increased volume.
D.Here, actually there is no technical change of state as only the distinction between the two states (liquid and gas) is not possible. So, this is not the correct option for this question.
Hence, option C is the correct answer for this question.
Note:
Above the critical point, condensation of a gas will never occur. It is possible to add a large amount of pressure to a gas in a closed container and it may become very thick, but it may not exhibit a meniscus. Molecules contain high kinetic energy at critical temperatures, and the intermolecular forces in the molecules are reduced as a result.
