Question
Question: A gas ‘X’ is present with saturated water vapour over water liquid at a total pressure of \(1.5{\tex...
A gas ‘X’ is present with saturated water vapour over water liquid at a total pressure of 1.5atm. Vapour pressure of H2O at same temperature is 0.5atm. What is the solubility of gas ‘X’ in terms of moles in 10 mole H2O.
A.1×10−3mole
B.5×10−2mole
C.2×10−3mole
D.1×10−2mole
Solution
To solve this question, you must recall Henry’s Law. Henry’s law states that the amount of gas dissolved in a liquid is proportional to its partial pressure above the liquid.
Formula used:
P α s
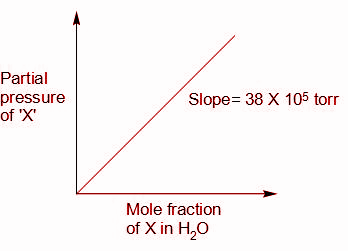
From the graph, we can write,
P=KHs
Where, sis the solubility of gas in moles per litre
KH is the Henry’s law constant, and KH=38×105torr=76038×105atm
P is the partial pressure of the gas in the mixture in atm
Complete step by step answer:
The total pressure of the system is given to be 1.5atm
And the vapour pressure of water is 0.5atm
Thus, the partial pressure of the gas will be 1atm
Using the formula, P=KHs
We can write it as, P=KHnH2OnX
⇒nX=KHP.nH2O
Substituting the values:
nX=1×38×105760×10
∴nX=2×10−3moles
Thus, the correct option is C.
Note:
Henry’s law has various applications.
It is used in production of carbonated beverages. Under high pressure, solubility of carbon dioxide gas increases. When the bottle is opened and it is exposed to atmospheric pressure, solubility of the gas decreases and the gas bubbles are released from the liquid.
At high altitude, due to low atmospheric pressure concentration of oxygen in the blood and tissues is very low and people feel weak and are unable to think properly.
It also has applications in underwater diving.
Gas can be breathed at ambient pressure which increases with increasing depth due to hydrostatic pressure. Solubility of gases increases at depth as per Henry's law, so the body tissues dissolve more oxygen over time till it is saturated for the depth. When ascending the diver is exposed to lower pressure conditions and the solubility of the oxygen dissolved in the tissues decreases as well. If the supersaturation is too great it can cause blockages in capillaries or distortion in the solid tissues.
