Question
Question: A gas when passes through \({{K}_{2}}C{{r}_{2}}{{O}_{7}}\) and dil. \({{H}_{2}}S{{O}_{4}}\) solution...
A gas when passes through K2Cr2O7 and dil. H2SO4 solution turns it green, the gas is:
A.CO2
B.NH3
C.SO2
D.Cl2
Solution
Potassium dichromate has an excellent property of oxidising other compounds. Chromium is present in the+4 oxidation state, where it itself gets reduced and the oxidation state changes to +3.
Complete answer:
In order to answer our question, we need to learn about potassium dichromate. The compound is prepared from chromite ore (FeO.Cr2O3). Sodium dichromate is filtered and then treated with dil. H2SO4. Being less soluble, sodium sulphate separates out. Then hot concentrated solution is cooled when red crystals of sodium dichromate separates out on standing and then a hot concentrated solution of sodium dichromate is treated with potassium chloride to get potassium dichromate.
Na2Cr2O7+2KCl→K2Cr2O7+2NaCl
The properties of potassium dichromate are:
i. It is an orange crystalline solid with melting point 670 K.
ii. It is appreciably soluble in hot water but moderately soluble in cold water.
iii. On heating K, Cr,o, decomposes to give potassium chromate and chromic oxide.
4K2Cr2O7+Δ→4K2Cr2O4+2Cr2O3+3O2
iv. Potassium dichromate acts as a strong oxidising agent in acidic medium.
v. When a mixture of metal chloride is heated with potassium dichromate and concentrated sulphuric acid, the orange red fumes of chromyl chloride are formed.
K2Cr2O7+4NaCl+6H2SO4→2KHSO4+4NaHSO4+2CrO2Cl2+3H2O
The structure of potassium dichromate is:
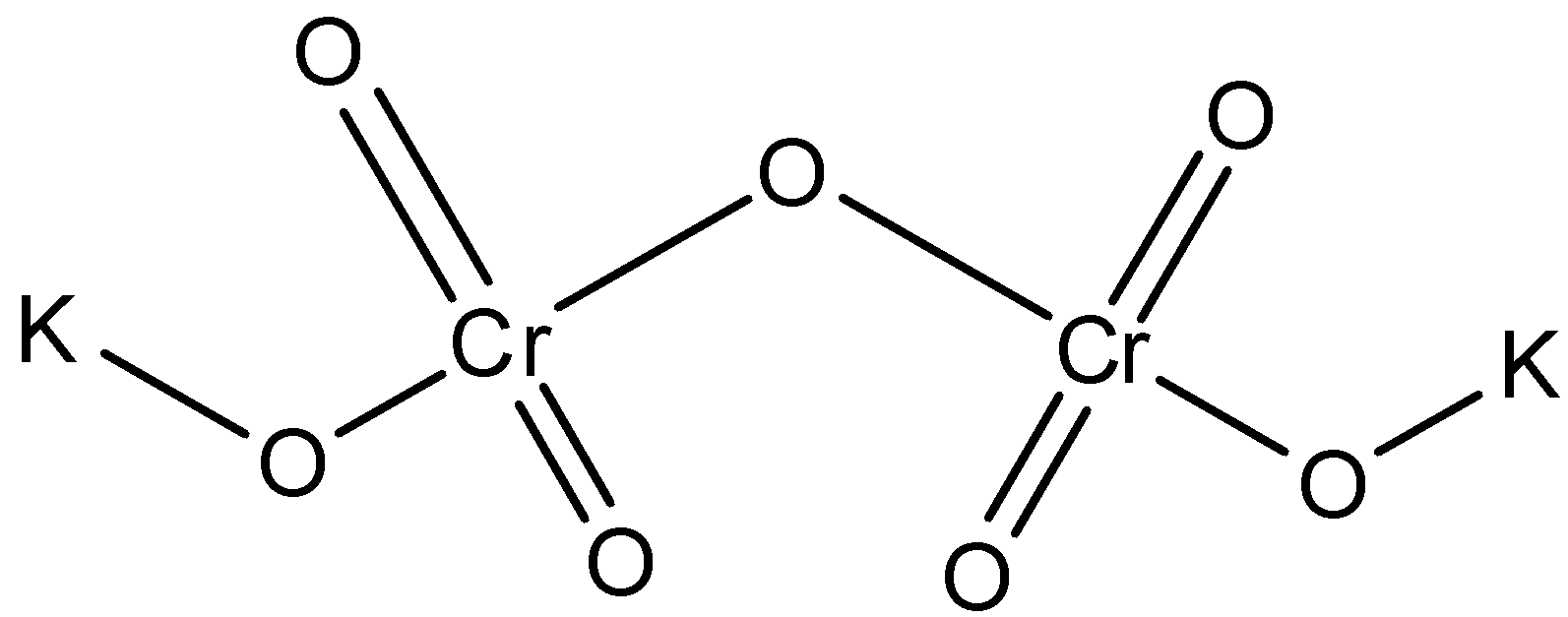
Now, let us come to our question. In the compound, chromium is present in the +4 oxidation state. However, when sulphur dioxide gas is passed through it, then the oxidation state of chromium changes to +3 and the colour of the solution changes to green. The reaction is represented as:
K2Cr2O7+2H2SO4+3SO2→2Cr2(SO4)3+K2SO4+H2O
So, we get the correct answer as option C.
NOTE: The uses of potassium dichromate are:
i.For the volumetric estimation of ferrous salts, halides and sulphides.
ii. For the preparation of other chromium compounds such as chrome alum chrome yellow etc
iii. Used in chrome tanning in leather industry
iv. Used as an oxidising agent.
