Question
Question: A gas is taken through the cycle A B C A, as shown. What is the net work done by the gas? 
A. 3000J
B. 1000J
C. Zero
D. 2000J
Solution
The product of pressure and volume is represented by an area on the p-v diagram. The area under this curve tells us the work done during a thermodynamic process. In this process it can be found out by calculating the area of the curve this is a simple and quick method.
The curve showing the variation of volume of a substance along the x-axis and the variation of the pressure along the y-axis is the P-V diagram.
In this question first, find the total change in volume along the x-axis and change in pressure along the y-axis and then find the work done under the curve.
Complete step by step solution:
Given that the gas is going through the curve ABCA
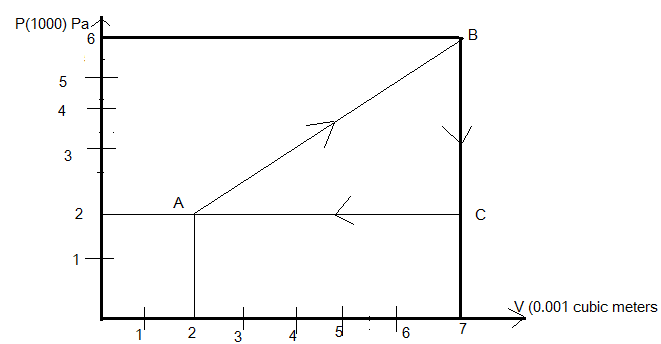
Where the change in volume takes place from 2×10−3m3to 7×10−3m3along positive x-axis and pressure changes from 2×105Pa to 6×105Pa along the y-direction.
Hence,
△V=5×10−3m3
△P=4×105Pa
So the area under the curve for the total work done will be equal to
Net work is done = area under the curve A B C
Hence the total work is done by the gas under the curve =1000J
Option (B) is correct.
Note: Students should be careful in some cases where in some cases specific volume is plotted along the x-axis instead of volume; in such cases area under the curve represents work per unit mass of the working fluid given as J/Kg.
