Question
Question: A gas is compressed isothermally to half its initial volume. The same gas is compressed separately t...
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then,
A. Compressing the gas isothermally will require more work to be done.
B. Compressing the gas through adiabatic process will require more work to be done
C. Compressing the gas isothermally or adiabatically will require the same amount of work
D. Which of the cases (whether compression through isothermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
Solution
You could make a PV diagram for the given compression of gas where it is being compressed into half its initial volume for under both the given processes. You may recall that the area under the PV diagram gives the work done in that process. Thus, you will be able answer the question from the plot.
Complete solution:
In the question, we said that a gas is being compressed isothermally to half its volume separately by isothermal process as well as adiabatic process. We are supposed to find the correct statement from the given options regarding the work done for both processes.
Let us plot the PV diagram for both the processes.
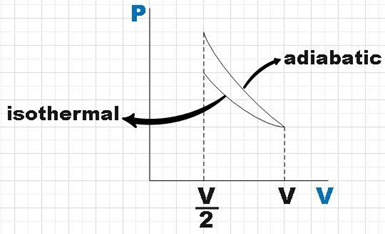
We see that the plot of the gas being adiabatically compressed is steeper than that of the plot for the gas being compressed isothermally. We know that the area under the PV diagram will give us the work done by the gas.
Without any doubt we could see that the area under the PV diagram for adiabatic process is more than that of the area under the plot for isothermal process which further implies that the work done for the gas to compress to half its volume by adiabatic process is more than that of the isothermal process.
Hence, option B is found to be the correct answer.
Note:
If you are wondering how we know that the PV diagram for adiabatic processes is steeper than isothermal, you could recall the relations for the respective processes. For isothermal process,
PV=k
But for adiabatic process,
PVγ=k
Clearly from these relations, the PV diagram for adiabatic process is steeper.
