Question
Question: A gas is allowed to expand at constant temperature from its initial volume of 400mL to a final volum...
A gas is allowed to expand at constant temperature from its initial volume of 400mL to a final volume of 2000mL. The final pressure is calculated to be 5 atm. Calculate its initial pressure.
Solution
Hint: As the initial and final volumes are given, along with the initial pressure, it is advised to think of a relation that relates initial and final pressures and volumes. In every thermodynamic system, the product remains constant.
Complete step by step solution:
In order to answer the question, let us know what Boyle’s Law is. According to Boyle’s law, in a thermodynamic system the pressure is always inversely proportional to the volume, given that the temperature remains constant, and vice versa. In simple terms, if pressure is increased, then volume will decrease.
Let us take the example of a piston to explain this. The best example of a piston is an injection. There is medicinal fluid inside the injection. Now, when the syringe is pushed, then it creates pressure on the fluid inside it. As the pressure increases, we see that the medicinal fluid reduces in volume. Finally, it spills out from the tip.
One more example is the cold drink bottle. Cold drinks are maintained under high pressure, but on opening, the pressure reduces. So, volume increases, and that is the reason sometimes cod drinks spill out during opening. Let us look at the graph between pressure and volume:
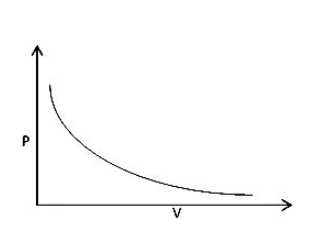
As we can see, the graph takes the shape of the equation y=x1.
Now, to solve the question, we will use the relation P1V1=P2V2, where the left side of the equation represents initial pressure and volume, right side measures the final values. So,
P1×400=5×2000P1=4005×2000=25atm
So, the initial pressure comes to be 25 atm , which is smaller than the final pressure, as volume is more. So, we obtain our answer at 25atm.
Note: During the application of Boyle’s law, temperature needs to be constant. Moreover, as both the initial and final volumes have their units same, in mL, so it is not required to convert them into SI, as their ratios will be same.
