Question
Question: A gas can be liquified by pressure alone only, when its temperature is ___________ its critical temp...
A gas can be liquified by pressure alone only, when its temperature is ___________ its critical temperature.
(A) more than
(B) less than
(C) less than or equal to
(D) equal to or higher than
Solution
Critical temperature of a given substance can be defined as the highest temperature value at which the substance can exist as in the liquid state. If the temperature above the critical temperature of the particular gaseous substance is reached then it can no longer be liquified, not even depending on the amount of pressure applied to it.
Complete step by step answer:
Let us check out what the Critical temperature is?
We can say that the critical temperature of a substance can be said to be the highest temperature at which the particular substance can exist as in the liquid state.
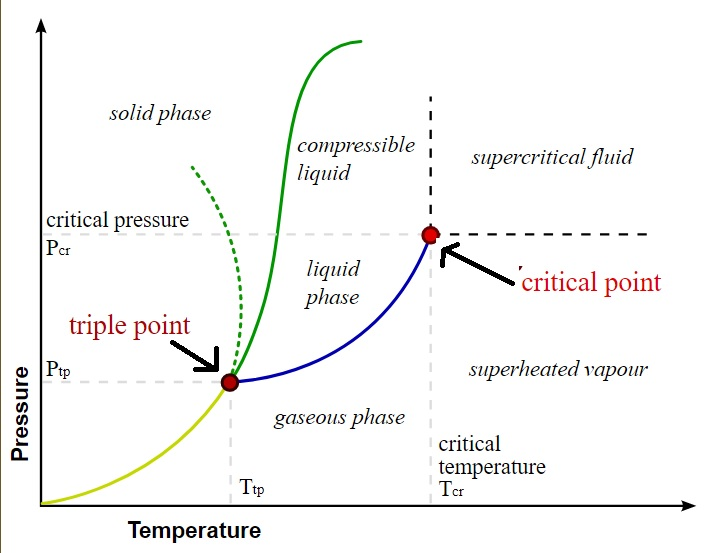
Let’s understand with the help of a graph.
This graph shows the triple point and the critical point of a substance.
The French physicist Charles Cagniard in the year 1822 discovered the critical point of a liquid.
Now you would be wondering what is a triple point? So, the triple point is the point at which a substance can be present in all three states of matter.
Pressure is plotted on the Y-axis and the temperature is plotted on the X-axis in the above graph. So, the critical temperature can thus be obtained from the value obtained from the X-axis corresponding to the critical point.
Now we will introduce a new term critical pressure and thus you would be wondering what is critical pressure? So, the critical pressure is the pressure that is required to liquefy a substance at critical temperature.
Therefore, the substance can be liquefied only when temp is less than or equal to the critical temperature of the given substance.
Therefore, the correct answer comes out to be ‘(C) less than or equal to’.
Note: You should conceptually be very careful about the different terms namely critical temperature, critical pressure and critical constants. Volume of one mole of the gas present at the critical temperature is said to be the critical volume and pressure present at this temperature is said to be critical pressure. The critical temperature, pressure and volume are also collectively called critical constants.
