Question
Question: For the series of bases shown below, rank the set from strongest to weakest....
For the series of bases shown below, rank the set from strongest to weakest.
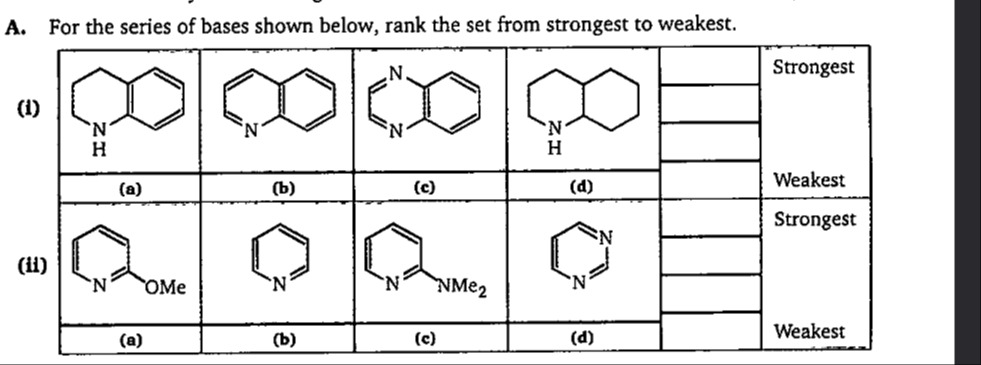
1,2,3,4-Tetrahydroisoquinoline
Isoquinoline
1,5-Naphthyridine
Decahydroquinoline
(d), (a), (b), (c)
Solution
For ranking the basicity of nitrogen-containing compounds, we consider the availability of the lone pair of electrons on the nitrogen atom to accept a proton. Factors influencing basicity include hybridization of the nitrogen atom, resonance effects, inductive effects, and steric hindrance.
Part (i): The bases are: (a) 1,2,3,4-Tetrahydroisoquinoline: Secondary amine, sp3 hybridized nitrogen, localized lone pair. (b) Isoquinoline: Aromatic heterocycle, sp2 hybridized nitrogen, delocalized lone pair. (c) 1,5-Naphthyridine: Aromatic heterocycle, sp2 hybridized nitrogens, delocalized lone pairs. (d) Decahydroquinoline: Saturated bicyclic secondary amine, sp3 hybridized nitrogen, localized lone pair.
General trend: Aliphatic amines (sp3 hybridized nitrogen with localized lone pair) are stronger bases than aromatic amines (sp2 hybridized nitrogen with delocalized lone pair). Therefore, (a) and (d) are stronger bases than (b) and (c).
Comparing (a) and (d): Both are secondary amines. Decahydroquinoline (d) is a fully saturated bicyclic system, while 1,2,3,4-tetrahydroisoquinoline (a) has a fused aromatic ring. Saturated amines are generally stronger bases. Experimental pKa values confirm that decahydroquinoline is a stronger base than 1,2,3,4-tetrahydroisoquinoline (pKa of conjugate acid ~10-11 for (d) vs 9.4 for (a)).
Comparing (b) and (c): Both are aromatic. Isoquinoline (b) has a pKa of its conjugate acid around 5.4. 1,5-Naphthyridine (c) has two nitrogen atoms that withdraw electron density from each other, making it a weaker base with pKa values around 3.1 and 1.1. Thus, isoquinoline is a stronger base than 1,5-naphthyridine.
Therefore, the ranking from strongest to weakest for part (i) is (d) > (a) > (b) > (c).
Part (ii): The bases are: (a) 2-Methoxypyridine: Pyridine ring with a methoxy group at the 2-position. Nitrogen is sp2 hybridized. Methoxy group has electron-donating resonance (+M) and electron-withdrawing inductive (-I) effects. (b) Pyridine: A basic aromatic heterocycle. Nitrogen is sp2 hybridized. (c) 2-(Dimethylamino)pyridine: Pyridine ring with a dimethylamino group at the 2-position. Nitrogen is sp2 hybridized. Dimethylamino group is a strong electron-donating group via resonance (+M effect). (d) Pyrimidine: Diazine with two nitrogen atoms in the aromatic ring, both sp2 hybridized.
Comparing the bases: Pyrimidine (d) is a weak base (pKa of conjugate acid ~1.3). Pyridine (b) is a moderately weak base (pKa of conjugate acid ~5.2). 2-Methoxypyridine (a): Methoxy group at the ortho position makes it a weaker base than pyridine (pKa of conjugate acid ~3.2). 2-(Dimethylamino)pyridine (c): Dimethylamino group is a strong electron-donating group, increasing electron density on the pyridine nitrogen, making it a much stronger base (pKa of conjugate acid ~9.6).
Therefore, the ranking from strongest to weakest for part (ii) is (c) > (b) > (a) > (d).
Answer
For part (i), the ranking from strongest to weakest is: (d), (a), (b), (c). For part (ii), the ranking from strongest to weakest is: (c), (b), (a), (d).
