Question
Question: A flask contains a mixture of isohexane and \({\text{3 - methylpentane}}\) . one of the liquids boil...
A flask contains a mixture of isohexane and 3 - methylpentane . one of the liquids boils at 600c and the other one boils at
630c . what is the best way to separate the two liquids and which one will be distilled out first?
A) fractional distillation, 3 - methylpentane
B) simple distillation, isohexane
C)fractional distillation, isohexane
D) simple distillation, 3 - methylpentane
Solution
Distillation is the process of separating components of a mixture based on different boiling points. It is used to separate a mixture of liquids, the liquid can be heated to force components, which have different boiling points, into the gas phase.
Complete Step by step answer: To separate two compounds having marginal difference in their boiling point, fractional distillation method is used
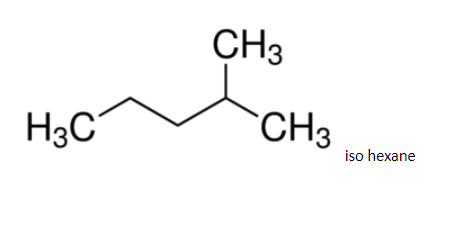
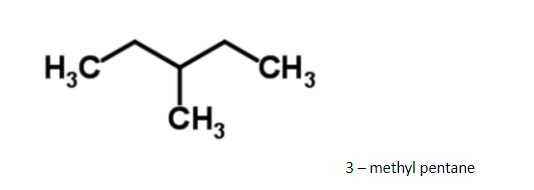
Since it has a regular structure, it will have a slightly higher boiling point.
Hence 3 - methylpentane, it boils at 630c. The difference in boiling points is marginal, so, fractional distillation method is used. The compound having lower boiling point distils out first. So, isohexane distils out first and the method used for separation of such liquids is fractional distillation.
Generally the component parts have boiling points that differ by less than 25∘C from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25∘C, a simple distillation is typically used.
Hence, option “C” is correct.
Note: Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize or boil.
