Question
Question: (a) Explain quinonoid theory of indicators. (b) Write notes on IUPAC conventions of representation...
(a) Explain quinonoid theory of indicators.
(b) Write notes on IUPAC conventions of representations of a cell.
Solution
(a) The theory is about the phenomenon of colour change during acid-base titrations. It is based upon the inter conversion of structures at equilibrium.
(b) It involves double vertical slashes to separate the oxidation and reduction halves. The oxidation half is always towards the left.
Complete step by step answer:
(a) We all are familiar with substances known as indicators. They give a visual signal when contacted with acid or base. The most common of them all is the litmus paper. These are not precise methods but give a rather vague report about a test subject. In experiments such as acid-base titrations, we need to determine its end point which has to be as accurate as possible. For this reason there are specific chemicals known as indicators that are very precise and easy to work with.
Indicators are mostly organic compounds which are weak acids or bases themselves. During an acid-base titration a change of colour in the conical flask kept below a burette is marked as its end. The Quinonoid theory proposes a mechanism that describes this “colour changing” process.
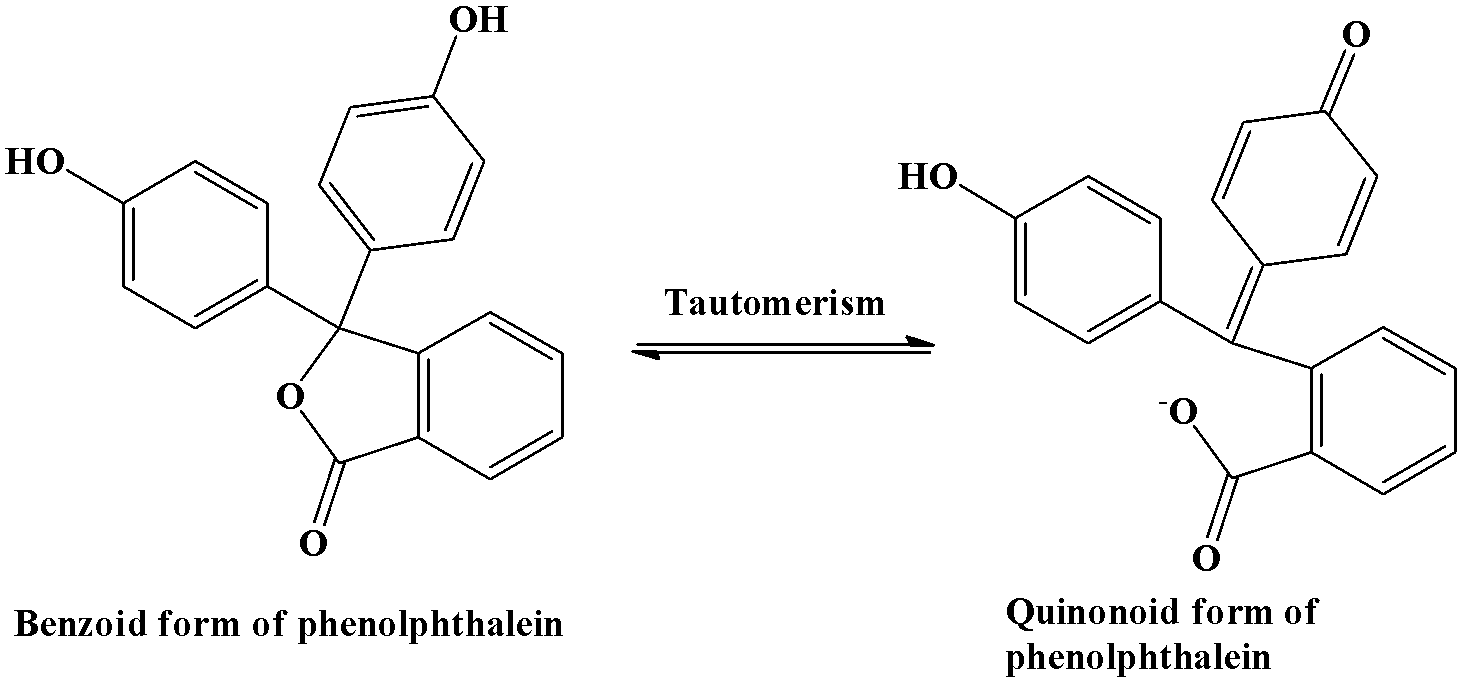
This theory states that the indicators have two tautomeric forms that happen to stay at equilibrium with each other. One of the forms is called the benzoid form and the other is called quinonoid form. One of the above mentioned forms is only stable in acidic solution while the other is only stable in basic solution. The change of colour occurs when the equilibrium shifts from one to another.
Let’s take the example of a common indicator “phenolphthalein”. Its benzoid form is stable in acidic medium and is colourless; while it’s quinonoid form is stable in basic medium and imparts a pink colour to the solution. The tautomeric conversion is shown below:
(b) The IUPAC conventions for representing a Galvanic cell are listed below:
- The oxidation half reaction should be present first followed by the reduction half reaction.
- The oxidation half reaction should be written as the non-oxidised state of atom and then the oxidised state separated by a vertical line. The reduction half reaction should be written as the reduced state of the atom followed by the non-reduced state separated by a vertical line.
- The two half reactions should be separated by two vertical lines.
- The physical states of the elements must be written.
- The reduction potential magnitude, if needed, must be written towards the right hand side of this representation.
Let’s take an example of the Daniel cell. It is a Galvanic cell meaning it converts chemical energy into electrical energy and is composed of Zinc and Copper.
The reduction half reaction is: Cu2+(aq)+2e−→Cu(s)
The oxidation half reaction is: Zn(s)→Zn2++2e−
This can be represented as: Zn(s)∣Zn2+(aq)∣∣Cu2+(aq)∣Cu(s)
Note: Tautomers are isomeric compounds. They have the same carbon skeleton but have different arrangements of hydrogen atoms. They are mostly in equilibrium with each other but this is not always the case.
This “∣∣ ” represents salt bridge while “∣ ” represents the interaction between the electrode and the electrolytic solution. Do not interchange them.
