Question
Question: (a) Draw the structures of the following molecules: \[ {\left( i \right){\text{ }}XeO{F_4}} \\...
(a) Draw the structures of the following molecules:
(i) XeOF4 (ii) H2SO4(b) Write the structural difference between white phosphorus and red phosphorus.
Solution
Lewis structures or Lewis dot diagrams represent the structures that depict the bonding between the atoms of a molecule as well as the lone pair of electrons which may exist in a molecule.
Complete solution
a. In order to draw the Lewis dot structure, you must calculate the number of valence shell electrons in the compound. Let us draw the structure of each molecule one by one.
(i) XeOF4
Number of valence shell electrons in XeOF4 can be calculated as follows:
Therefore XeOF4 has 42 valence shell electrons. These valence electrons are allotted to the elements such that each element attains octet. But in the present case, Xenon is exceptional since it can expand its octet. Thus, the structure of XeOF4 is shown below:
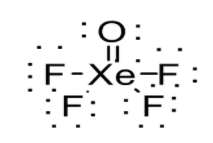
(ii) H2SO4
Number of valence shell electrons inH2SO4 can be calculated as follows:
Therefore H2SO4 has 32 valence shell electrons. If you divide this by 2 i.e. 232=16 number of electron pairs which are either shared by elements via bonds or lone pairs of electrons in the Lewis Structure. In the structure, line as well as pair dots denote the electron pair. If you count these both (line and pair of dots), you would see 16. Hydrogen can possess a maximum of 2 electrons, whereas other elements can possess a maximum of 8. Hydrogen atoms can be attached to the outside of oxygen molecules. The structure of H2SO4 is shown below:
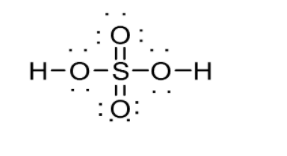
(b) The structural difference between white phosphorus and red phosphorus is stated below: White phosphorus consists of P4 molecules. Thus, white phosphorus exists as a P4 molecule in both solid as well as vapour states. On the other hand, the crystal structure of red phosphorus possesses a complicated network of bonding (it is polymeric) as it occurs as a chain of tetrahedral P4 units.
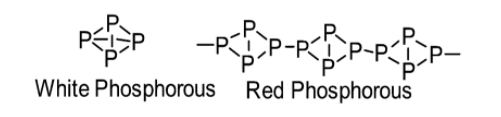
Note:
The main purpose to draw a Lewis dot structure is to determine the lone pair of electrons in molecules which helps to identify the formation of chemical bonds. Lewis structures can be drawn for molecules containing the covalent bonds as well as for coordination compounds.
