Question
Question: (a) Draw the structures of the following molecules: (i) \({{\text{N}}_{\text{2}}}{{\text{O}}_{\tex...
(a) Draw the structures of the following molecules:
(i) N2O5
(ii) HClO4
(b) Explain the following observations:
(i) H2S is more acidic than H2O.
(ii) Fluorine does not exhibit any positive oxidation state.
(iii) Helium forms no real chemical compound.
Solution
Draw the Lewis structures using the total valence electron count. We know that the acidic character depends on the electronegativity of the bond.
Complete answer:
(a)(i) Draw the structures of N2O5:
Calculate the valence electrons of N2O5 molecule as follows:
The valence electrons of N are 5 and the valence electrons of O are 6. Thus,
Valence electrons of N2O5 =(2×Valence electrons of N)+(5×Valence electrons of O)
=(2×5)+(5×6)
= 10 + 30
Valence electrons of N2O5 =40
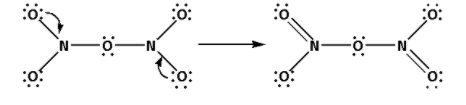
Draw the Lewis structure of N2O5 as follows:
The structure of N2O5 is,
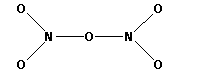
As six bonds are formed, twelve electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons = 40 - 12 = 28
Place the remaining 28 electrons around the oxygen atoms such that all the oxygen atoms complete their octets.
Thus, the structure of N2O5 is as follows:
(ii) Draw the structures of HClO4:
Calculate the valence electrons of HClO4 molecule as follows:
The valence electrons of H are 1, the valence electrons of Cl are 7 and the valence electrons of O are 6. Thus,
Valence electrons of HClO4
=(1×Valence electrons of H)+(1×Valence electrons of Cl)+(4×Valence electrons of O)
=(1×1)+(1×7)+(4×6)
= 1 + 7 + 24
Valence electrons of HClO4 =32
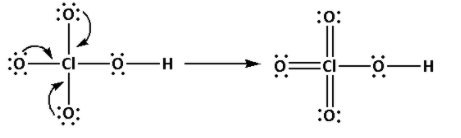
Draw the Lewis structure of HClO4 as follows:
The structure of HClO4 is,
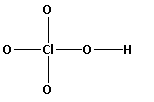
As five bonds are formed, ten electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons = 32 - 10 = 22
Place the remaining 22 electrons around the oxygen atoms such that all the oxygen atoms complete their octets.
Thus, the structure of HClO4 is as follows:
(b)(i) Explain why H2S is more acidic than H2O:
In H2S, the bond is formed between hydrogen and sulphur and in H2O bond is formed between hydrogen and oxygen.
The electronegativity of oxygen is more than that of sulphur. Thus, the bond dissociation enthalpy of the bond between hydrogen and sulphur is lower than that of the bond between hydrogen and oxygen.
Thus, H2S is more acidic than H2O.
(ii) Explain why fluorine does not exhibit any positive oxidation state:
The size of fluorine is very small and thus, electrons in fluorine are strongly attracted by the nuclear charge.
Thus, the removal of electrons is not possible from the atom.
Thus, fluorine does not exhibit any positive oxidation state.
(iii) Explain why helium forms no real chemical compound:
The electronic configuration of helium is 1s2.
The maximum capacity of the s orbital is 2 electrons.
Thus, the valence orbital of helium is completely filled.
Also, helium has high ionisation enthalpy and more positive electron gain enthalpy.
Thus, helium forms no real chemical compound.
Note: An atom can form chemical compounds when its valence orbital can accommodate electrons. In case of helium, valence orbital is completely filled and thus, it cannot form any chemical compounds.
