Question
Question: (a) Draw the structure of the following: i. \[Xe{F_2}\] ii \(Br{F_3}\) (b) Write the structure...
(a) Draw the structure of the following:
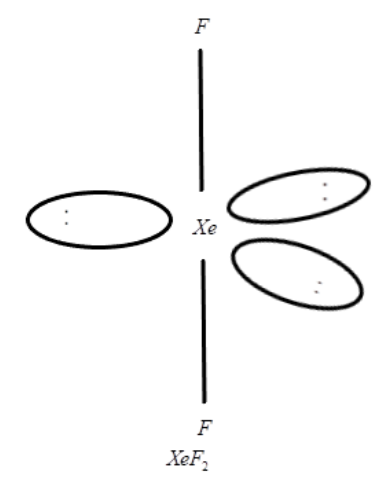
i. XeF2
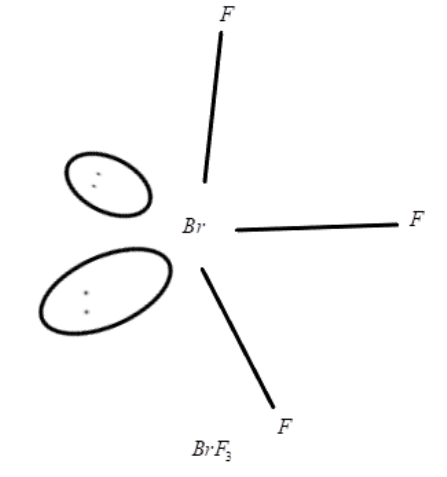
ii BrF3
(b) Write the structure difference between white phosphorus and red phosphorus.
Solution
As we know that the general electronic configuration of inert gases are ns2np6 except helium. Among the noble gas compounds, Xenon is known to form a number of compounds with electronegative elements like Fluorine and Oxygen. Phosphorus possesses three allotropic forms namely red, black and white.
Complete step by step answer:
(a)Let us recall some of the basic chemical properties of the noble gases like they possess the completely filled valence shell, high ionization enthalpy and more positive electron gain enthalpy. Xenon along with members of the group like helium, krypton, argon, neon and radon are termed as noble gas compounds. Among these Xenon is known to form a number of compounds with electronegative elements like Fluorine and Oxygen. Krypton forms very few compounds and only KrF2 is known. Even there are no true compounds of Argon, helium and Neon yet known.
i. Now let us talk about the structure of XeF2 which is a linear molecule and according to VSEPR theory, there are three non-bonding electrons pair around the equatorial region and also xenon prepared in 1:2 ratio reacts with fluoride ions at 673K and 1bar of pressure and results in the preparation of XeF2. We can explain this with a chemical reaction and can also make the structure of it:
Xe(g)+F2673K,1barXeF2(s)
ii. Now, let us talk about the structure of BrF3(Bromine trifluoride) which is an interhalogen compound and is soluble in sulphuric acid and a very well known fluorinating agent. It is a T-shaped molecule and planar where the bromine center possesses two electron pairs. It is formed when Bromine reacts with fluorine at a temperature of 20∘C. We can explain it by the reaction:
BrF3+3F220∘C2BrF3
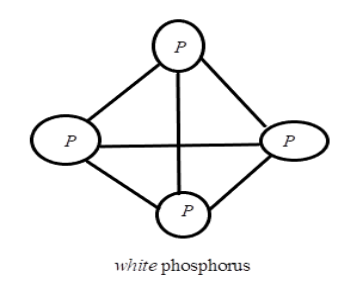
(b) As we have discussed, phosphorus consists of three important allotropic forms which are Red phosphorous, White phosphorous and Black phosphorus. We will talk about White phosphorus which consists of discrete tetrahedral P4 molecules. When compared to other solid phases, it is more reactive due to the angular strain in P4 molecules with an angle of 60∘. White phosphorus dissolves in a boiling NaOH solution in an inert atmosphere giving phosphine, PH3. The structure is as:
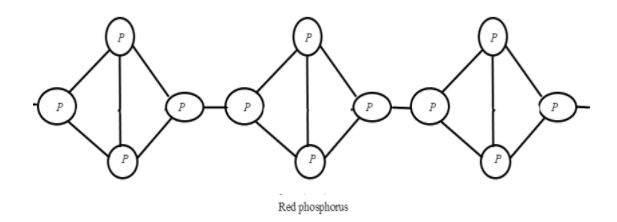
Whereas the Red phosphorus consisting of chains of P4 tetrahedra linked together is polymeric in nature. It is chemically much less reactive than white phosphorus and when it is heated under high pressure it results in a series of phases of Black phosphorus. The structure can be drawn as:
Note:
Try to remember the reagent in chemistry that is the basic need to be as good as anyone else in chemistry. And learn about the angles and configurations of the compound to make sure that if they have unpaired electrons or paired electrons which will be helpful in their bonding with other elements.
