Question
Question: (a) Draw the labelled diagram of Daniel cell. (b) Write the half-reactions of oxidation and reduct...
(a) Draw the labelled diagram of Daniel cell.
(b) Write the half-reactions of oxidation and reduction taking place on electrodes.
Solution
Recollect the difference between an electrolytic cell and galvanic cell. Where does oxidation take place in a galvanic cell? Think about the direction of electron flow in a galvanic cell.
Complete answer:
Galvanic cell is an electrochemical cell which converts chemical energy to electrical energy. Some examples include dry cell, fuel cell, Ni-Cd battery, lead storage cell.
Daniel cell is a galvanic cell in which a spontaneous chemical reaction takes place to produce electricity. It consists of two half cells. One half cell is a beaker containing a strip of metallic zinc dipped in 1M aqueous zinc sulphate solution. The second half cell consists of a beaker having a metallic strip of copper immersed in 1M aqueous copper sulphate solution. The two solutions are connected with the help of a salt bridge containing saturated solution of KCl in agar agar gel.
Daniel cell can be schematically represented as,
Zn∣ZnSO4(0.1M)∥CuSO4(0.1M)∣Cu
Diagrammatically Daniel cell can be represented as:
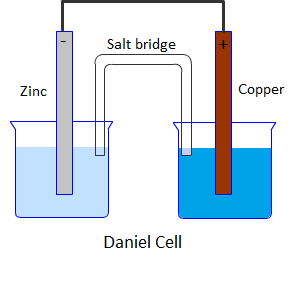
Half-cell reactions:
-At Anode (Oxidation half-cell):
Zn(s)→Zn2++2e−
-At Cathode (Reduction half-cell):
Cu2++2e−→Cu(s)
Note: Don’t get confused with charge on anode and cathode in electrolytic cell and galvanic cell. Remember LOAN-Left Oxidation Anode. In electrolytic cells, positively electrode is anode and negatively charged electrode is cathode. In galvanic cells, it is opposite. Positively charged electrode is a cathode and a negatively charged electrode is anode.
