Question
Question: (a) Draw structure of the following compounds: 1\. \(Xe{F_4}\) 2\. \({N_2}{O_5}\) (b) Write th...
(a) Draw structure of the following compounds:
1. XeF4
2. N2O5
(b) Write the structural difference between white phosphorus and red phosphorus.
Solution
A structural formula displays the atoms of the molecule in the order they are bonded. It can tell us about the central atom and the atoms bonded to it. White and Red Phosphorus are the two allotropes of Phosphorus. Allotropes are the different physical forms of an element in which it can exist. They are different structural modifications of the element.
Complete step by step answer:
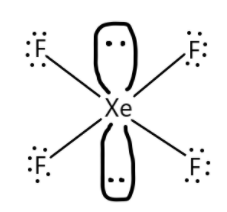
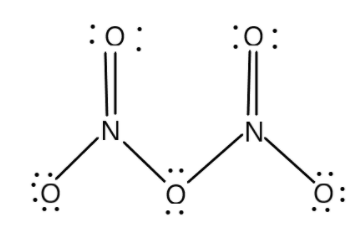
(a) A structural formula displays the atoms of the molecule in the order they are bonded. It also depicts how the atoms are bonded to one another for example single, double or triple bonds. For example in XeF4 we have one atom of Xenon and four atoms of fluorine. We can get from this information that the central atom is xenon and fluorine is bonded with the central atom. XeF4 is also known as Xenon Tetrafluoride and is the first discovered binary compound of noble gas. N2O5 is also called dinitrogen pentoxide and is one of the binary nitrogen oxides.
The structure of XeF4 is as shown below:
The structure of N2O5 is as follows:
(b) Before finding the difference between white and red phosphorus we need to know both of them in detail.
White phosphorus also called yellow phosphorus is simply tetraphosphorus ( P4 ). It exists as molecules made up of four atoms in a tetrahedral structure. This tetrahedral arrangement results in ring strain and instability. The molecule is described as consisting of six single P-P bonds. Two different crystalline forms are known, the α and the β form. The α form is defined as the standard state of the element but is actually metastable under standard conditions. It has a body-centred cubic crystal structure which transforms into β form at 195.2K . The β form is believed to have a hexagonal crystal structure. It is a translucent waxy solid that quickly becomes yellow when exposed to light and therefore it is also called yellow phosphorus. It is toxic and glows greenish in dark and is highly flammable and self-igniting upon contact with air. It is also slightly soluble in water.
Red phosphorus is of the most common allotropes of phosphorus and is a derivative of P4 molecule. It exists in an amorphous network of phosphorus atoms. It is more stable than white phosphorus. It has a deep red colour and powdery texture. It is formed when white phosphorus is kept under certain conditions. It is odourless and not poisonous to humans.
The main difference between them is that white phosphorus is highly reactive and exists as a P4 molecule but red phosphorus is less reactive and exits as a chain of P4 units.
Note:
XeF4 is also known as Xenon tetrafluoride and is the first discovered binary compound of noble gas. N2O5 is also called dinitrogen pentoxide and is one of the binary nitrogen oxides. White and Red Phosphorus are the two allotropes of Phosphorus. Allotropes are the different physical forms of an element in which it can exist. They are different structural modifications of the element.
